Improving UV-C stability in polypropylene through synergistic phenolic or hydroxylamine-based additives with UV absorbers
Marcos Vinícius Basaglia , Jessica Caroline Ferreira Gimenez
, Jessica Caroline Ferreira Gimenez , Manoel Gustavo Petrucelli Homem
, Manoel Gustavo Petrucelli Homem , Sandra Andrea Cruz
, Sandra Andrea Cruz , Lucas Henrique Staffa
, Lucas Henrique Staffa , Sílvia Helena Prado Bettini
, Sílvia Helena Prado Bettini
 , Jessica Caroline Ferreira Gimenez
, Jessica Caroline Ferreira Gimenez , Manoel Gustavo Petrucelli Homem
, Manoel Gustavo Petrucelli Homem , Sandra Andrea Cruz
, Sandra Andrea Cruz , Lucas Henrique Staffa
, Lucas Henrique Staffa , Sílvia Helena Prado Bettini
, Sílvia Helena Prado BettiniVol. 19., No.2., Pages 124-139, 2025
DOI: 10.3144/expresspolymlett.2025.10
DOI: 10.3144/expresspolymlett.2025.10
GRAPHICAL ABSTRACT

ABSTRACT
UV-C radiation (200–280 nm) is recognized for its effectiveness in disinfection, but it also induces degradation in polymeric materials such as polypropylene (PP), reducing their service life. Stabilizing additives are a viable approach to mitigating or delaying the degradation process. However, the chemical groups within these additives may adversely affect stabilization under UV-C exposure due to their potential to absorb the radiation. This study investigates the degradation of PP under UV-C radiation and evaluates the performance of stabilization systems containing phenolic antioxidants (Irganox 1010), hydroxylamine (Irgastab FS 042), and UV absorbers (Tinuvin 1577). PP films were exposed to UV-C radiation for 24, 48, and 96 h, corresponding to doses of 1000, 2000, and 4000 J/cm2. Degradation was assessed using size exclusion chromatography (SEC), parallel plate rheometry, infrared spectroscopy (FTIR), static water contact angle, and mechanical testing. The independent use of antioxidants or UV absorbers resulted in reduced carbonyl group formation relative to neat PP, but these were insufficient to prevent PP brittleness after 96 h of exposure. In contrast, the combined use of hydroxylamine or phenolic additives with UV absorbers effectively preserved PP ductility, allowing deformations exceeding 300% without fractures, indicating a synergistic effect.
RELATED ARTICLES
Nikita V. Eremin, Svetlana Y. Voronina, Taisiya A. Shalygina, Valery V. Vlasov, Semyon A. Fesik, Anna A. Sukhanova
Vol. 20., No.2., Pages 168-185, 2026
DOI: 10.3144/expresspolymlett.2026.14
Vol. 20., No.2., Pages 168-185, 2026
DOI: 10.3144/expresspolymlett.2026.14
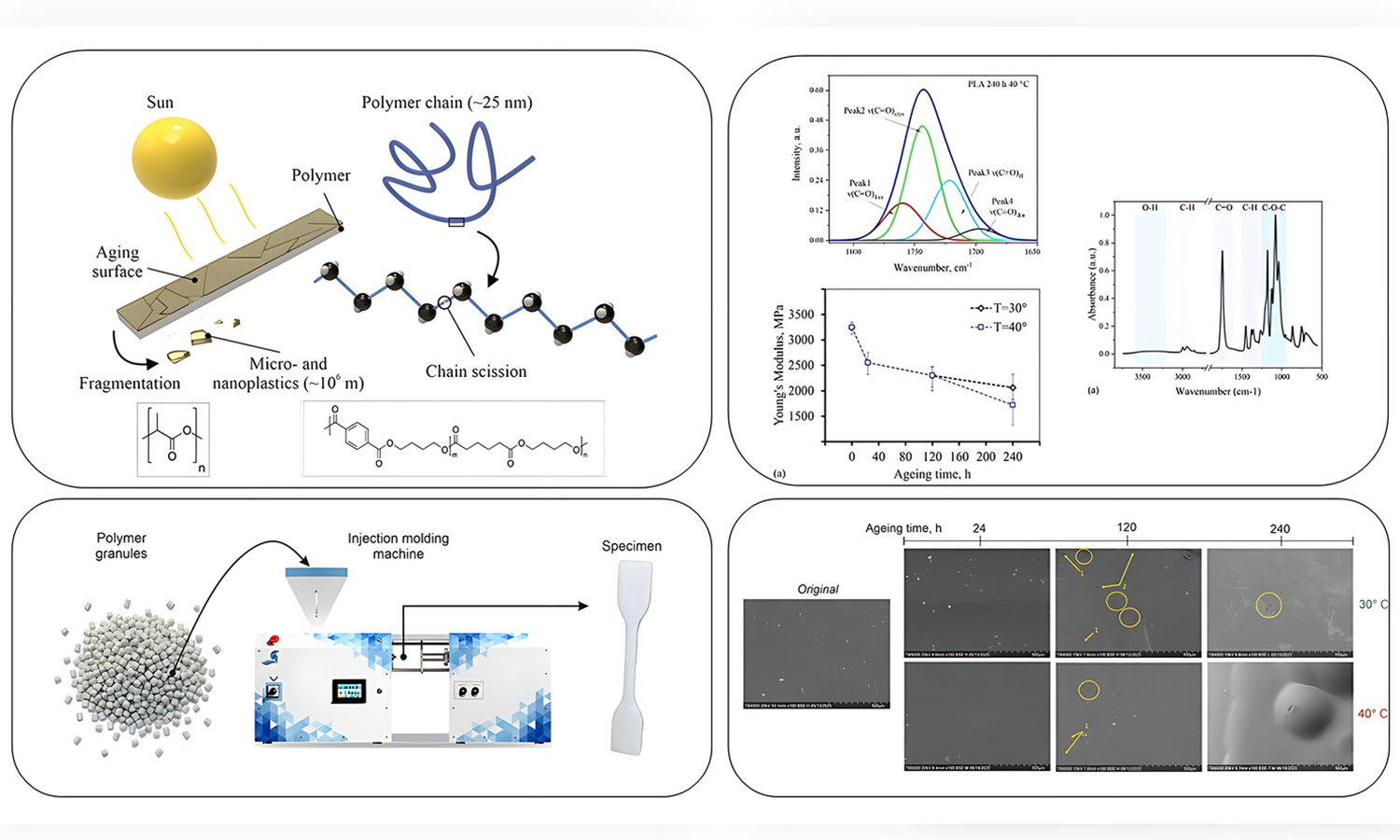
This article is devoted to the study of the degradation of two widely used biodegradable polymers, polylactic acid (PLA) and poly(butylene adipate-co-terephthalate) (PBAT), which are in demand in packaging, medicine, and agriculture. The effect of ultraviolet radiation (UV) at elevated temperatures on polymer ageing was investigated for 24, 120, and 240 h using a specially designed setup. Scanning electron microscopy, Fourier transform infrared spectroscopy, and tensile testing were employed to provide a comprehensive assessment of changes. PLA showed rapid degradation: after 120 h, its surface developed cracks and voids, and at 240 h, it became heavily damaged with cavities. PBAT degraded more gradually: at 240 h, large cracks and cavities were observed. For PLA, early ageing led to a shift and broadening of the carbonyl band, reflecting disorder and ester scission. For PBAT, a decrease in the intensity of the carbonyl shoulder and a slight shift of the main peak at elevated temperature indicated phase redistribution and the formation of new functional groups. Mechanically, PLA exhibited a sharp loss of strength and ductility in the first day of ageing, while PBAT showed greater stability, with slower reductions in stiffness and strength but a strong temperature-dependent decline in elongation. These findings are important for guiding the design of biodegradable polymers with improved durability.
Kankanok Longkaew, Philippe Daniel, Taweechai Amornsakchai, Thanchanok Ratvijitvech, Pranee Phinyocheep
Vol. 19., No.8., Pages 843-859, 2025
DOI: 10.3144/expresspolymlett.2025.64
Vol. 19., No.8., Pages 843-859, 2025
DOI: 10.3144/expresspolymlett.2025.64
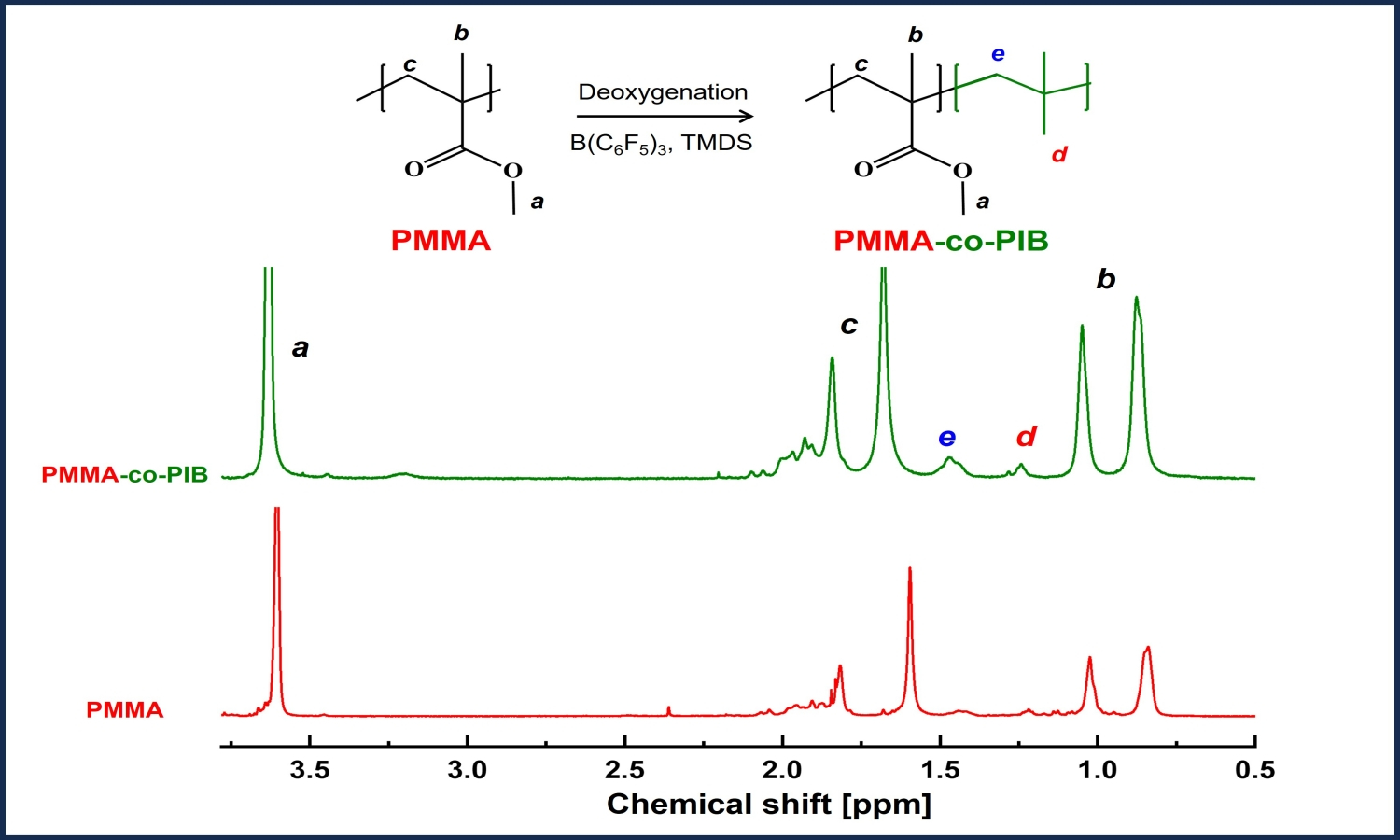
Poly(methyl methacrylate) or PMMA is a versatile material because of its ease of manipulation and biocompatibility. However, brittleness limits its applications; hence, copolymerization of PMMA with rubber such as polyisobutylene (PIB) is required. PMMA-co-PIB preparation by conventional copolymerization requires the purification of co-monomers under an inert atmosphere. In this study, the transformation of PMMA into PMMA-co-PIB was explored using a deoxygenation reaction. PMMA was treated with tris(pentafluorophenyl) borane and 1,1,3,3-tetramethyldisiloxane (TMDS) at room temperature, 50, and 70 °C for 1, 2, and 3 day reaction times. FTIR results revealed a reduction in the ester functional group of PMMA at 1724 cm–1. PMMA was partially converted into PIB moieties, forming PMMA-co-PIB. The highest degree of deoxygenation (48.2%) was obtained after a reaction for 1 day at room temperature. When the PIB structure was present in the PMMA backbone, the PMMA glass transition temperature (115.6 °C) was shifted to a lower temperature (83.7°C). A significant decrease in the modulus of PMMA from 3.40 to 1.29 GPa (PMMA-co-PIB) reveals improved toughness. This straightforward post-polymerization approach involving the deoxygenation of PMMA offers a promising, simple, and mild condition for synthesizing PMMA-co-PIB. This will enable new applications in medical appliances or high-value coating materials.



