Revisiting the polymerization of 2-[N-Boc-5-aminopentyl]-2-oxazoline: Toward amino-functionalized poly(2-oxazoline)s via microwave-assisted synthesis
Marcelina Bochenek , Barbara Mendrek
, Barbara Mendrek , Wojciech Wałach
, Wojciech Wałach , Agnieszka Kowalczuk
, Agnieszka Kowalczuk , Alicja Utrata-Wesołek
, Alicja Utrata-Wesołek , Agnieszka Fus-Kujawa, Natalia Oleszko-Torbus
, Agnieszka Fus-Kujawa, Natalia Oleszko-Torbus
 , Barbara Mendrek
, Barbara Mendrek , Wojciech Wałach
, Wojciech Wałach , Agnieszka Kowalczuk
, Agnieszka Kowalczuk , Alicja Utrata-Wesołek
, Alicja Utrata-Wesołek , Agnieszka Fus-Kujawa, Natalia Oleszko-Torbus
, Agnieszka Fus-Kujawa, Natalia Oleszko-Torbus
Vol. 19., No.12., Pages 1226-1237, 2025
DOI: 10.3144/expresspolymlett.2025.91
DOI: 10.3144/expresspolymlett.2025.91
GRAPHICAL ABSTRACT
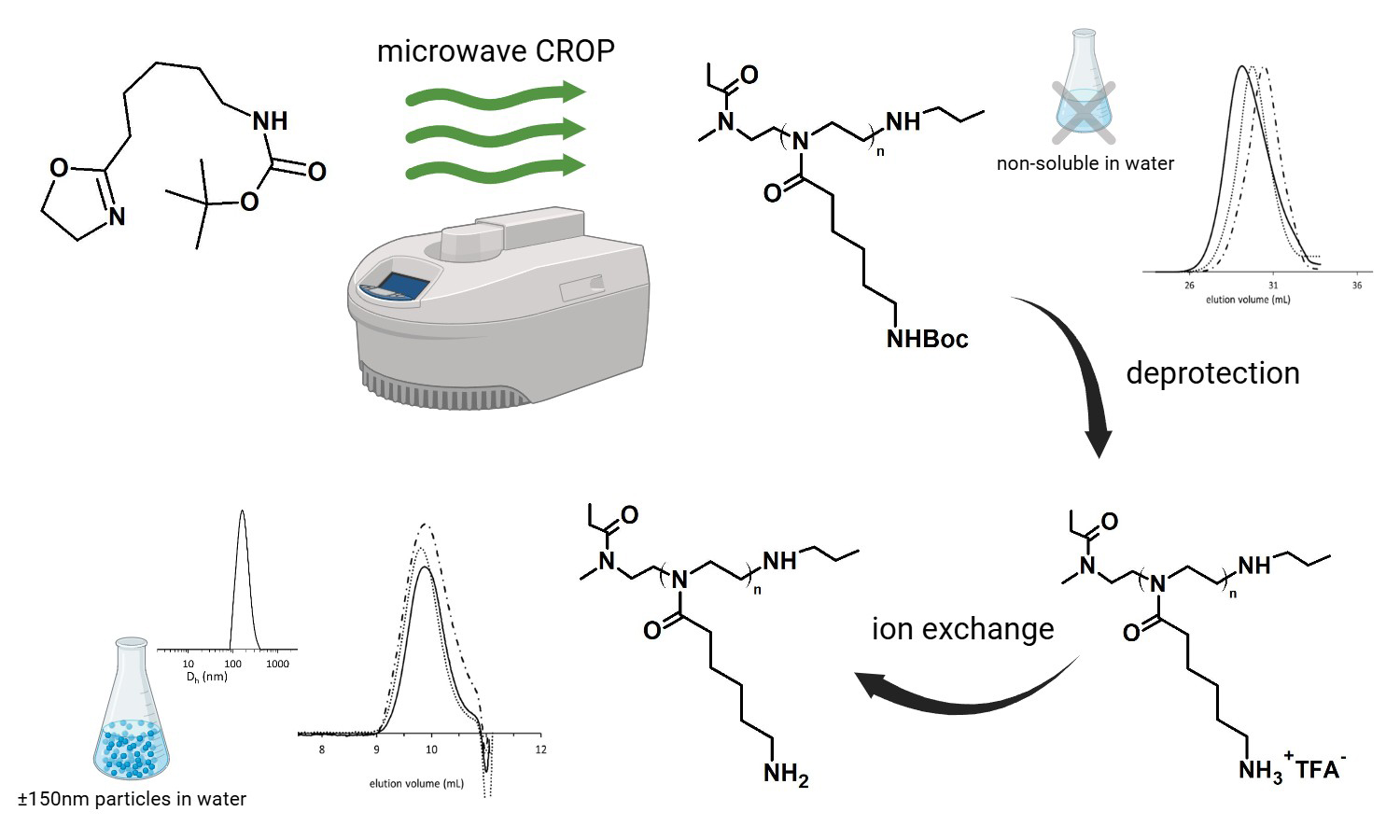
ABSTRACT
In this study, a series of novel homopolymers based on N-Boc-protected 2-(aminopentyl)-2-oxazoline (P(PentNHBocOx)) were synthesized via microwave-assisted cationic ring-opening polymerization (CROP), achieving high monomer conversions (up to 100%) and low dispersity values (Ð = 1.08–1.30). The resulting polymers were quantitatively deprotected using TFA/DCM, followed by ion exchange to yield amino-functionalized poly(2-oxazoline)s (P(PentNH2Ox)). Comprehensive characterization of the polymers confirmed successful deprotection without polymer degradation, preservation of low dispersity, and the formation of nanoscale aggregates in aqueous solutions falling within the size range of 140–152 nm, suitable, for biomedical applications. Cytotoxicity assays demonstrated that the polymers are non-toxic to human dermal fibroblasts, maintaining cell viability above 70% even at higher concentrations and extended incubation times. These findings highlight the potential of P(PentNH2Ox) as promising, biocompatible candidates for biomedical and nano-therapeutic applications.



