Synthesis and evaluation of poly(isoprene-co-acrylonitrile) as synthetic rubber with enhanced oil resistance
Vol. 17., No.10., Pages 1042-1055, 2023
DOI: 10.3144/expresspolymlett.2023.78
DOI: 10.3144/expresspolymlett.2023.78
GRAPHICAL ABSTRACT
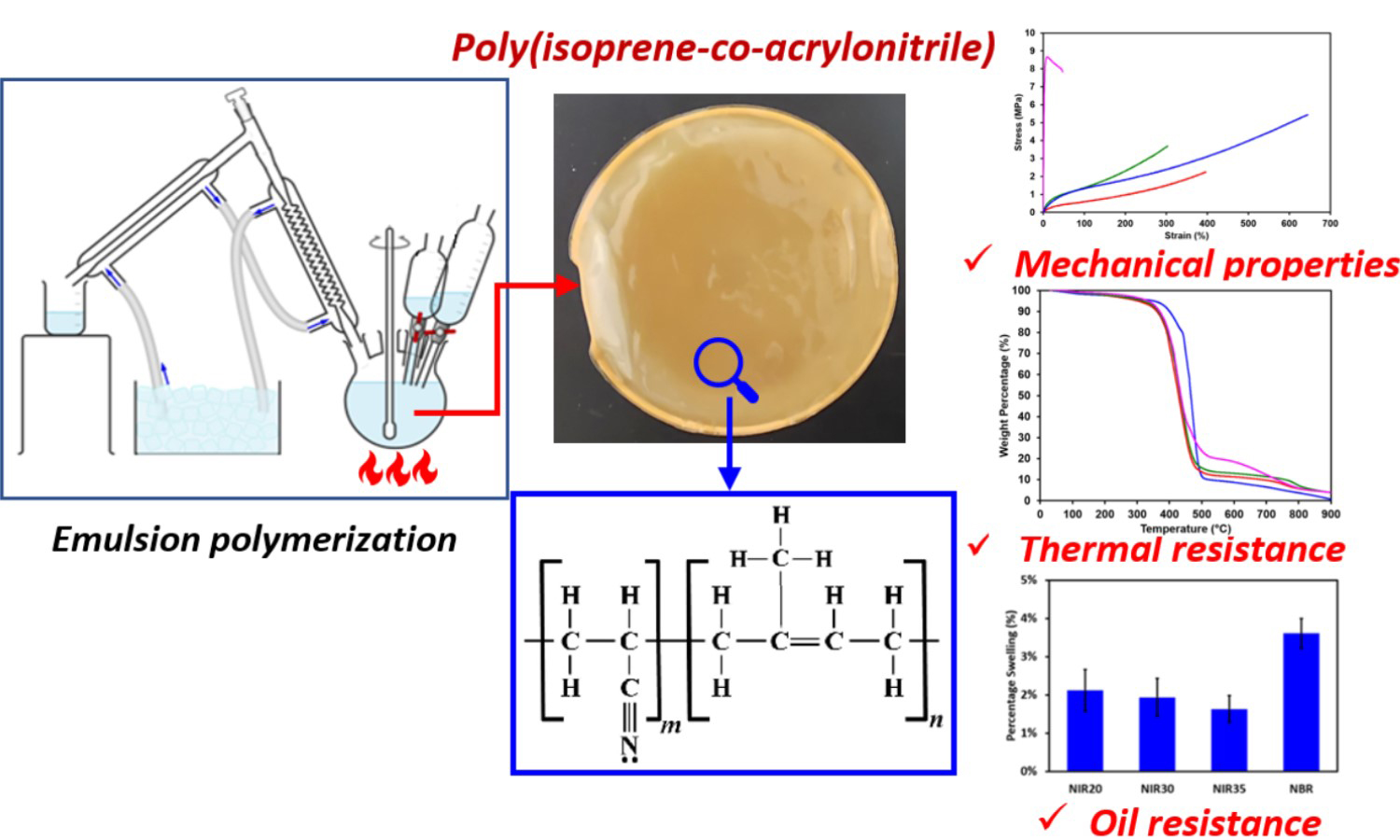
ABSTRACT
Increasing demand for durable rubber and rapid advancement in the automotive sector has made oil-resistant rubber an increasingly important material. Among them, nitrile rubber (NBR) is the most iconic due to its extraordinary oil resistance contributed by the polar nitrile pendant groups. Synthesis of NBR, however, is highly hazardous due to the explosive nature of the gaseous monomer butadiene. In this work, poly(isoprene-co-acrylonitrile) (NIR) was synthesized using free radical emulsion polymerization, with liquid monomer isoprene as the diene in the rubber formulation. The spectroscopic analysis confirmed the formation of NIR and indicated that the polymerized isoprene in the rubber is predominantly of 1,4-microstructure. A series of rubbers with different contents of acrylonitrile were produced and the mechanical, thermal, as well as oil resistant property of the resultant rubber films were evaluated. Vulcanized NIR films displayed glass transition temperatures from –9.4 to 20.2 °C, suggesting that the polymers are rubbery at ambient and higher temperatures. The NIR rubber films exhibited excellent oil resistance with less than 2% swelling in mineral oil, good thermal stability with onset degradation temperature in the range of 361.3 to 369.9 °C, and adequate mechanical strength from 1.61 to 8.23 MPa. The synthesized NIR rubber has the potential to serve as an alternative to NBR which had been traditionally used for oil resistance applications.
RELATED ARTICLES
Kinsuk Naskar
Vol. 19., No.10., Pages 977-978, 2025
DOI: 10.3144/expresspolymlett.2025.73
Vol. 19., No.10., Pages 977-978, 2025
DOI: 10.3144/expresspolymlett.2025.73

This is an editorial article. It has no abstract.
Sirithorn Kaewklum, Parisa Faibunchan, Apinya Krainoi, Banyat Cherdchim, Jutharat Intapun
Vol. 19., No.9., Pages 929-945, 2025
DOI: 10.3144/expresspolymlett.2025.70
Vol. 19., No.9., Pages 929-945, 2025
DOI: 10.3144/expresspolymlett.2025.70
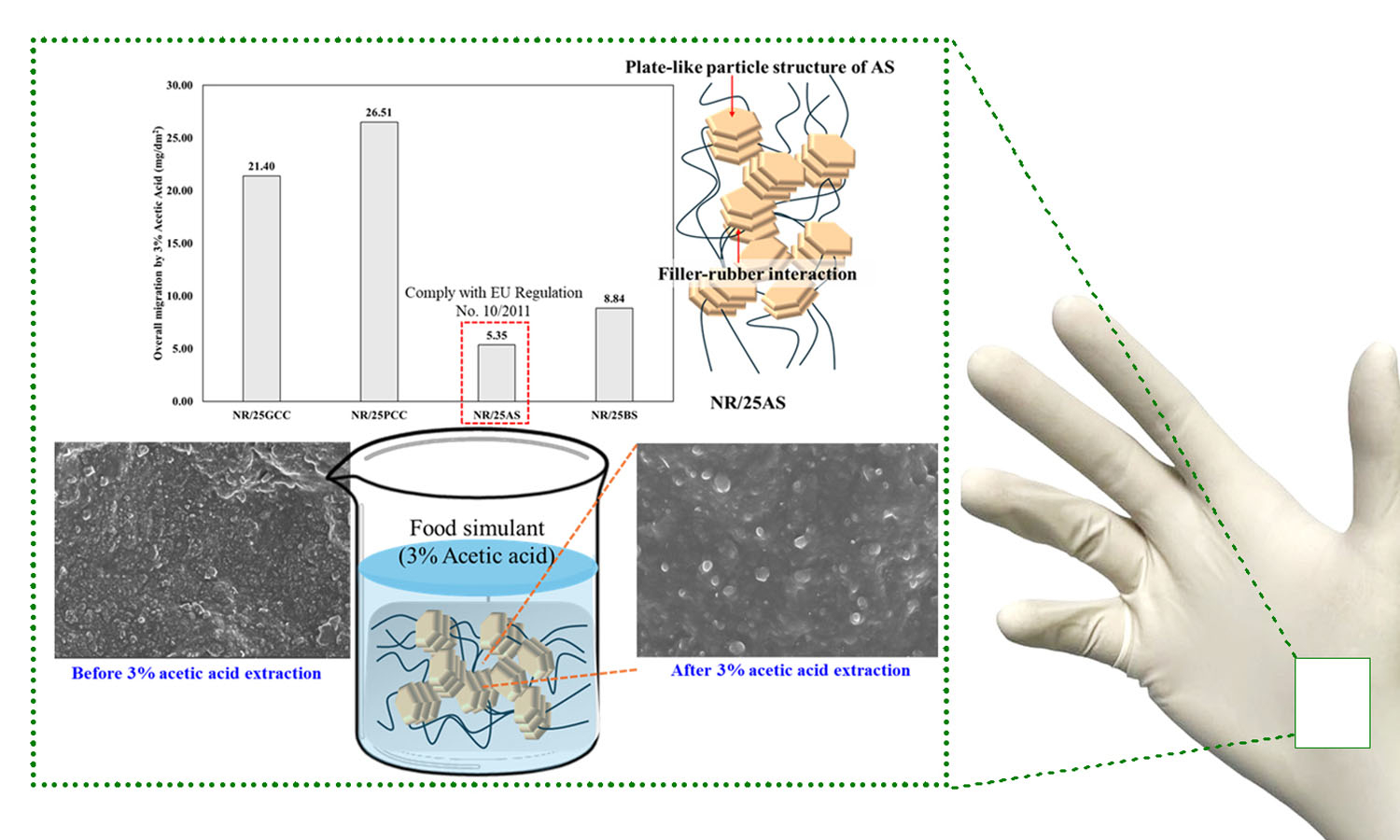
Powder-free natural rubber gloves for chemical migration resistance of food-contact grade are prepared using a variety of fillers, including ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), aluminum silicate (AS), and barium sulfate (BS)-filled natural rubber (NR), respectively. The properties of NR gloves, including mechanical, dynamic mechanical, and thermal properties, were investigated. Furthermore, the overall migration test of NR gloves was conducted according to the regulations for food contact gloves (EU Regulation No. 10/2011), using 3% acetic acid as the simulant. Among the fillers studied, the plate-like particles of AS facilitated the most effective filler-rubber interactions and reinforcement in AS-filled natural rubber (NR/AS). Consequently, the highest crosslink density, force at break, and damping properties of NR gloves were achieved by applying AS in the NR matrix. Moreover, the lowest overall migration level was observed for NR/AS with a value of 5.35 mg/dm2, which complies with EU Regulation (overall migration of food simulants shall not exceed 10 mg/dm2). Therefore, NR gloves filled with AS are suitable for food-contacting NR gloves.
Michaela Džuganová, Radek Stoček, Marek Pöschl, Ján Kruželák, Andrea Kvasničáková, Ján Hronkovič, Jozef Preťo
Vol. 18., No.12., Pages 1277-1290, 2024
DOI: 10.3144/expresspolymlett.2024.95
Vol. 18., No.12., Pages 1277-1290, 2024
DOI: 10.3144/expresspolymlett.2024.95
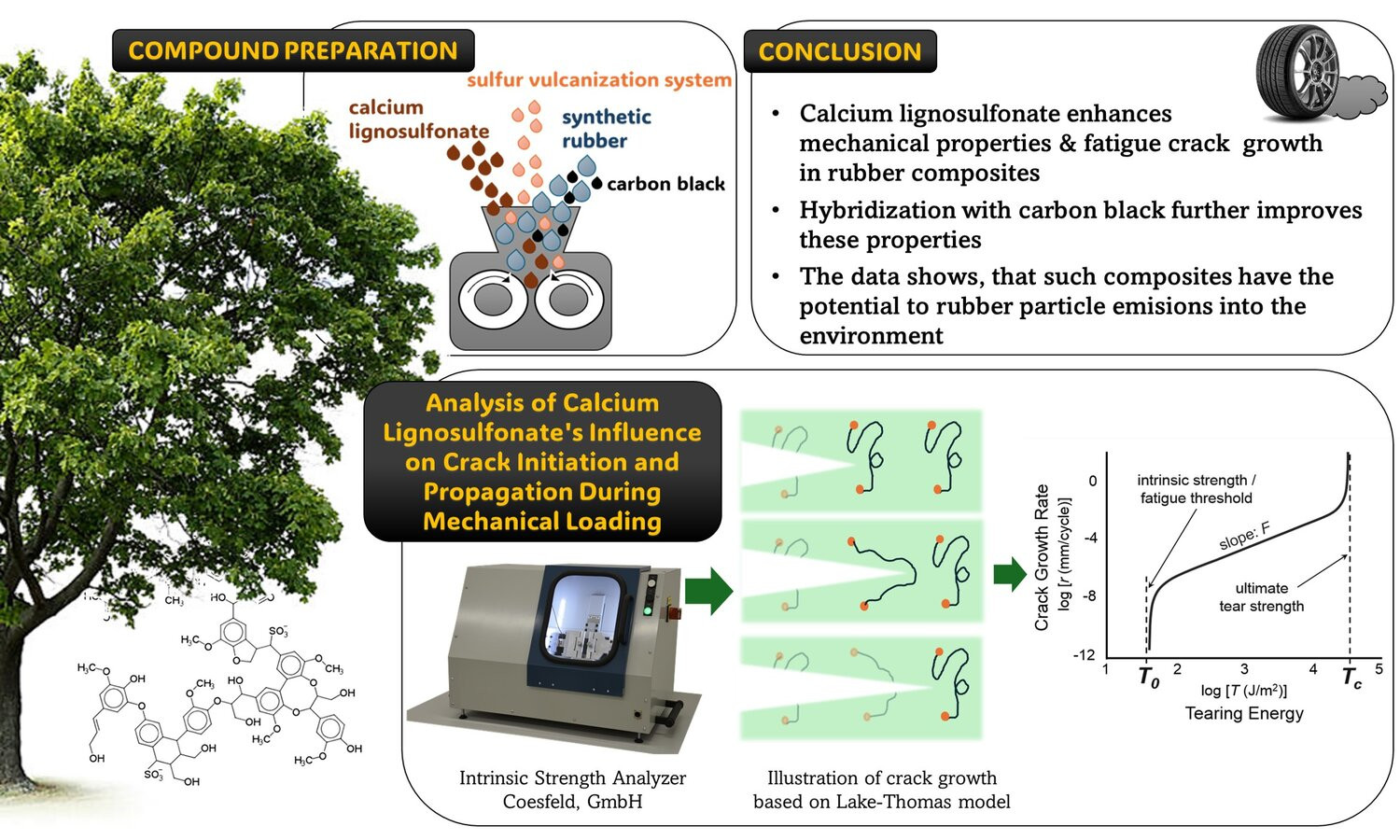
This study explores the transformative potential of calcium lignosulfonate (CaL) as a sustainable additive in rubber composites based on nitrile rubber (NBR) and styrene-butadiene rubber (SBR). Through comprehensive mechanical testing, fatigue crack growth (FCG) analysis, and scanning electron microscopy (SEM), we evaluated the tensile strength, elongation at break, surface morphology, and crack growth behavior of these innovative composites. By incorporating CaL into carbon black-reinforced rubber compounds (RUB/CB) based on nitrile rubber and styrene-butadiene rubber, we achieved good dispersion of both components as well as satisfactory morphology, resulting in tensile strengths of 16.3 and 12.7 MPa, respectively. While the CB/CaL hybrid did not significantly influence the intrinsic strength of the rubber samples, the ultimate strength of these compounds increased drastically – over five-fold compared to RUB/CB – indicating great potential for real-life applications. This study underscores the promise of lignin-based additives in the development of eco-friendly, highperformance rubber materials.
Ivy Gan, Wen Shyang Chow, Siong Hui Khoo, Mohamad Danial Shafiq
Vol. 18., No.7., Pages 689-704, 2024
DOI: 10.3144/expresspolymlett.2024.51
Vol. 18., No.7., Pages 689-704, 2024
DOI: 10.3144/expresspolymlett.2024.51
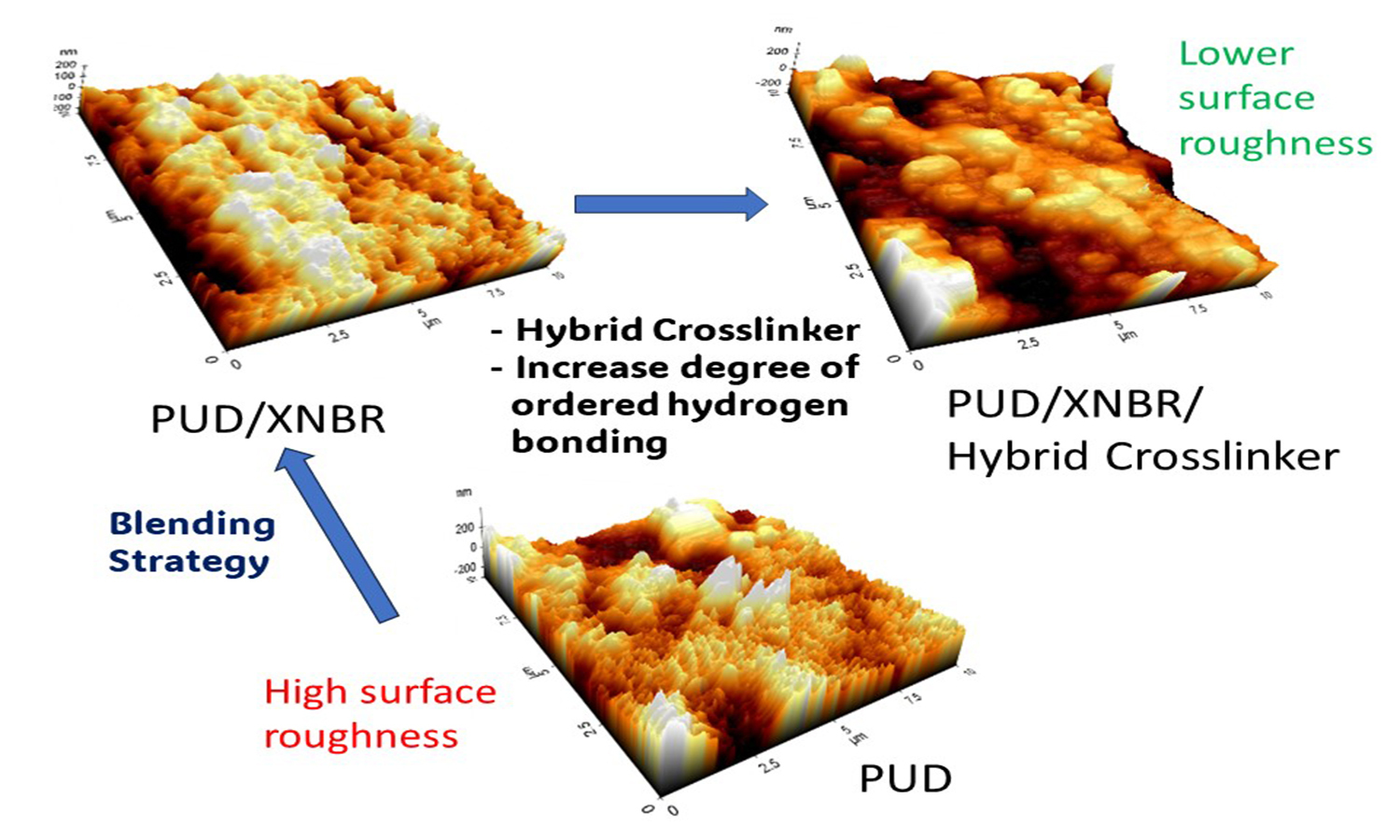
This study focuses on producing polyurethane dispersion (PUD)/carboxylated nitrile butadiene rubber (XNBR) blends with different types of crosslinkers without both accelerators and sulphur. Two types of crosslinkers: epoxide crosslinker and organo-modified siloxane, are introduced in the PUD/XNBR (blending ratio of 80:20). The zeta potential and particle size of the PUD/XNBR blends were determined using a dynamic light-scattering nanoparticle analyser. The chemical interaction and surface roughness of the PUD/XNBR blends were evaluated using Fourier transform infrared spectroscopy (FTIR) and atomic force microscopy (AFM). The zeta potential and particle size of the PUD were influenced by XNBR blending and the types of crosslinkers. FTIR observations indicate that the XNBR and the crosslinkers facilitated intermolecular hydrogen bonding and different extents of ordered hydrogen bonding. A higher degree of ordered hydrogen bonding can be associated with a higher surface roughness of the PUD/XNBR blends. Nevertheless, the hybrid crosslinkers can be used to achieve reasonable surface roughness for the easier donning of latex gloves. The research findings can be applied to design glove products with desirable surface roughness and intermolecular bonding.




