The influence of crosslink characteristics on key properties of dynamically cured NR/PP blends
Vol. 18., No.5., Pages 487-503, 2024
DOI: 10.3144/expresspolymlett.2024.36
DOI: 10.3144/expresspolymlett.2024.36
GRAPHICAL ABSTRACT
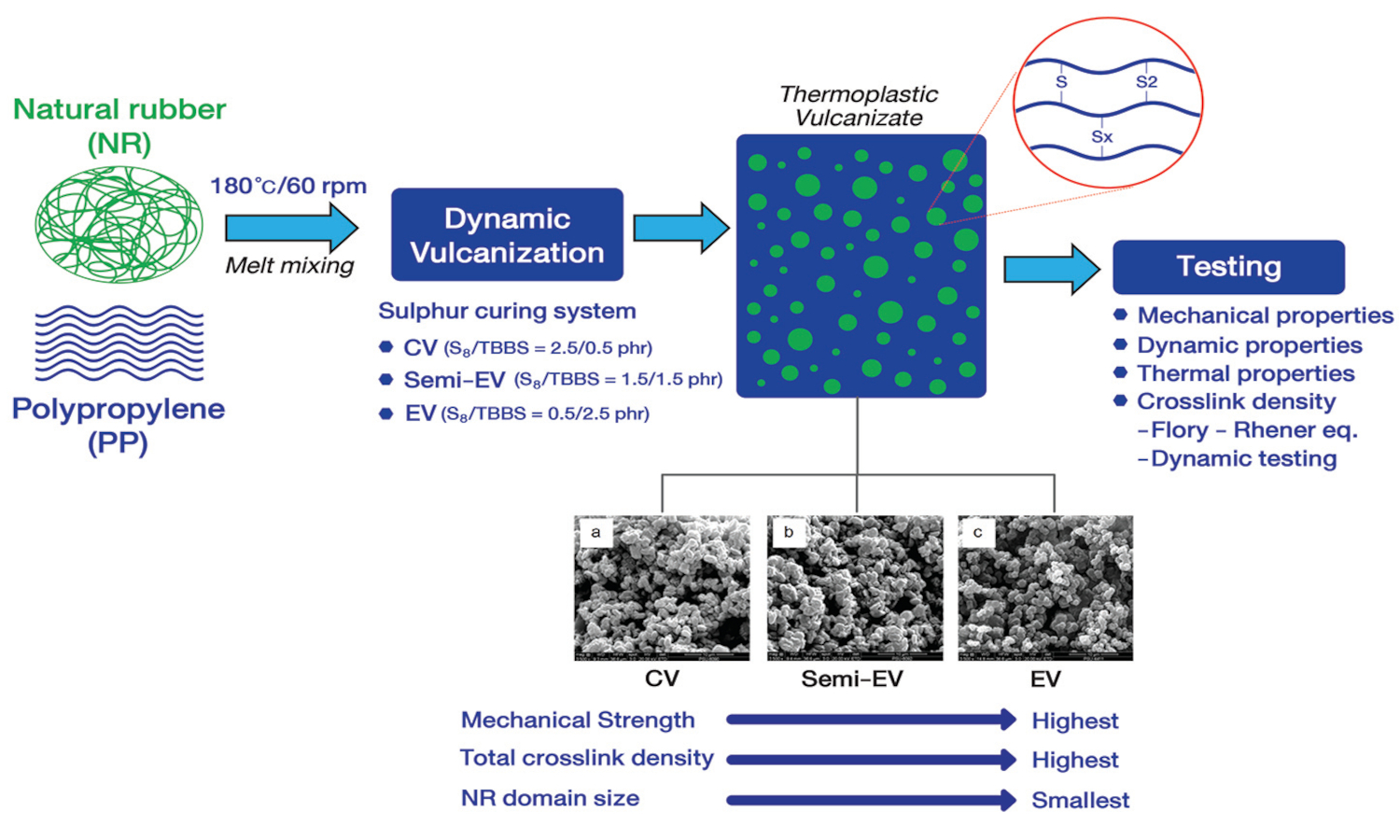
ABSTRACT
Thermoplastic
vulcanizates (TPVs) were prepared by blending natural rubber (NR) and
polypropylene (PP) using dynamic sulfur curing systems with varying
accelerator/sulfur ratios: 0.5/2.5, 1.5/1.5, and 2.5/0.5 phr, categorized as
conventional (CV), semi-efficient (semi-EV), and efficient (EV). The onset of
dynamic vulcanization closely corresponded with scorch time in statically cured
NR compounds. Mixing torque decreased over time, reflecting reversion patterns
in static curing. The CV system exhibited the highest reversion tendency due to
polysulfide linkage breakdown, forming stronger but shorter crosslinks. Dynamic
vulcanization induced higher reversion than static curing, influenced by shear
and extensional forces. Curing systems caused crosslinking rates, reversion,
and crosslink density and distribution variations. Unlike statically cured NR,
PP-extracted TPVs exhibited an inverse trend in total crosslink densities and
distributions; TPVs primarily comprised shorter crosslinks with opposed total
crosslink densities ranked EV > semi-EV > CV. This trend is strongly
correlated with superior mechanical strength, toughness, storage modulus,
viscosity, and rubber elasticity in the EV-cured TPV. EV system also had the
smallest vulcanized NR domains in the PP matrix.
RELATED ARTICLES
Azizon Kaesaman, Tassaneeya Khunrang, Charoen Nakason
Vol. 19., No.8., Pages 753-772, 2025
DOI: 10.3144/expresspolymlett.2025.58
Vol. 19., No.8., Pages 753-772, 2025
DOI: 10.3144/expresspolymlett.2025.58
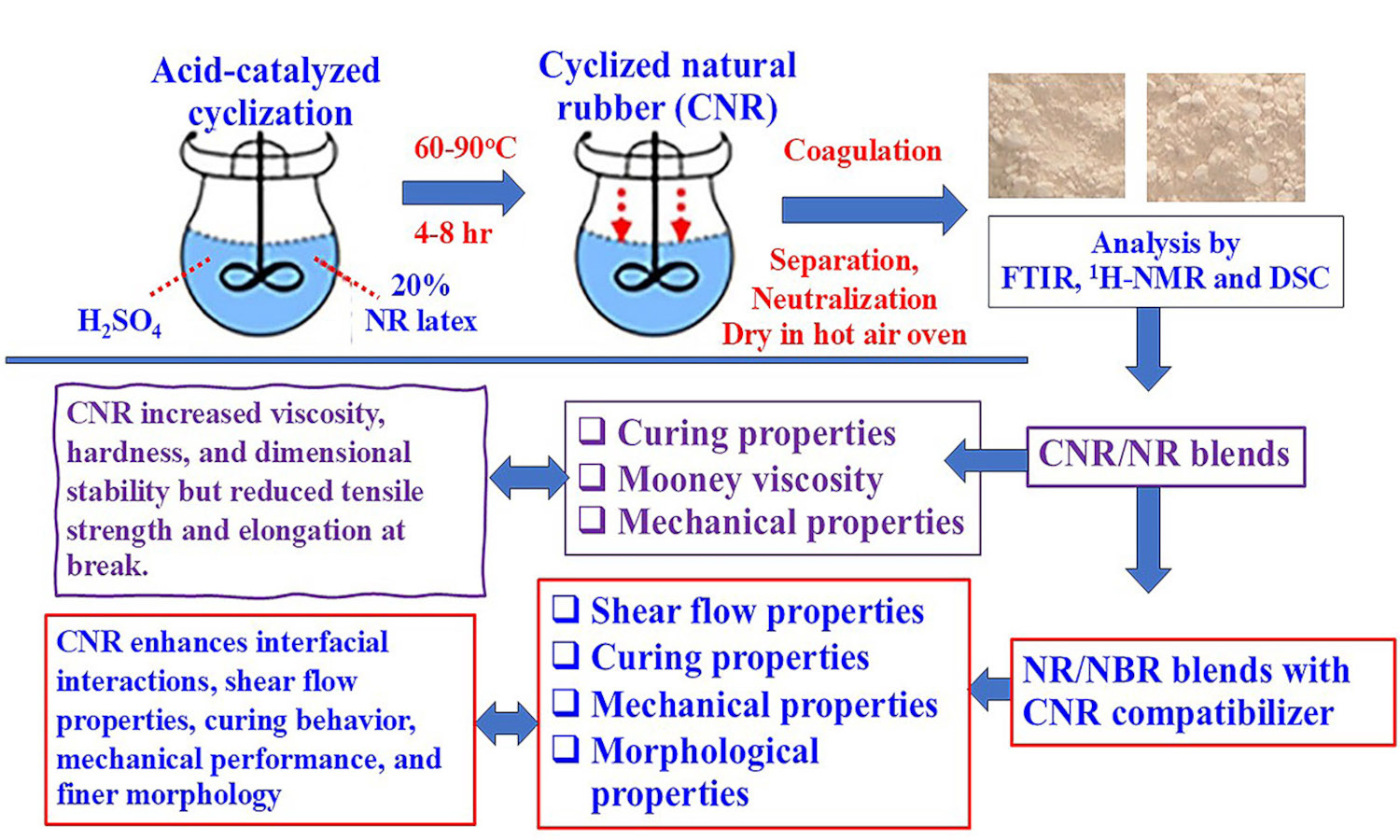
Cyclized natural rubber (CNR) was synthesized through the acid-catalyzed reaction of natural rubber (NR) latex using sulfuric acid as a catalyst and stabilized with a non-ionic surfactant. Cyclization was evaluated by iodine numbers under varying reaction times, temperatures, and NR-to-acid ratios. Fourier transform infrared spectroscopy (FTIR) and proton nuclear magnetic resonance spectroscopy (1H-NMR) confirmed the formation of cyclic structures in CNR molecules. Differential scanning calorimetry (DSC) showed that the glass transition temperature (Tg) of CNR increased with cyclization, indicating greater rigidity and less chain flexibility. CNR was then blended with NR and used as a compatibilizer in NR/acrylonitrile butadiene rubber (NBR)blends. It increased blend viscosity, hardness, and dimensional stability but reduced tensile strength and elongation due to its rigid cyclic domains. In NR/NBR blends, CNR outperformed a commercial homogenizer in enhancing interfacial interactions, leading to superior shear flow properties, curing behavior, and mechanical performance. This is attributed to the polar groups in CNR, which enhance intermolecular interactions and phase compatibility, resulting in finer phase morphology. This study highlights the potential of CNR as a versatile material for enhancing the performance of rubber compounds, with promising applications in advanced industrial formulations.
Liu Yang, Xuan Zhao, Sun Xinyu, Shuai Yuan, Lei Zhu
Vol. 19., No.8., Pages 783-795, 2025
DOI: 10.3144/expresspolymlett.2025.60
Vol. 19., No.8., Pages 783-795, 2025
DOI: 10.3144/expresspolymlett.2025.60

Waste tire rubber poses significant environmental challenges due to its non-biodegradability and complex crosslinkedvstructure. In this sense, this study aims to examine the utilization of deep eutectic solvents (DESs) in the desulfurizationvprocess of ground tire rubber (GTR). A range of hydrogen bond donors (HBDs), including ethylene glycol, malonic acid,vimidazole, toluene sulfonic acid, and urea, were combined with choline chloride, which serves as a hydrogen bond acceptorv(HBA), to synthesize deep eutectic solvents. Subsequently, these DESs are used in the modification of rubber devulcanizationvprocesses. Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and Horikx analysis werevused to confirm the occurrence of devulcanization. The studies confirmed that the devulcanization process was selective invnature, effectively reducing random chain scission while maintaining the integrity of the polymer. Furthermore, the vulcanizatesvobtained post-treatment demonstrated enhanced properties, including increased tensile strength, modulus, tear strength, hardness, and durability, with ethylene glycol-based DES (DES-E) exhibiting the most pronounced enhancements.
Abdulhakim Masa, Nureeyah Jehsoh, Sawitree Dueramae, Nabil Hayeemasae
Vol. 19., No.7., Pages 653-669, 2025
DOI: 10.3144/expresspolymlett.2025.50
Vol. 19., No.7., Pages 653-669, 2025
DOI: 10.3144/expresspolymlett.2025.50
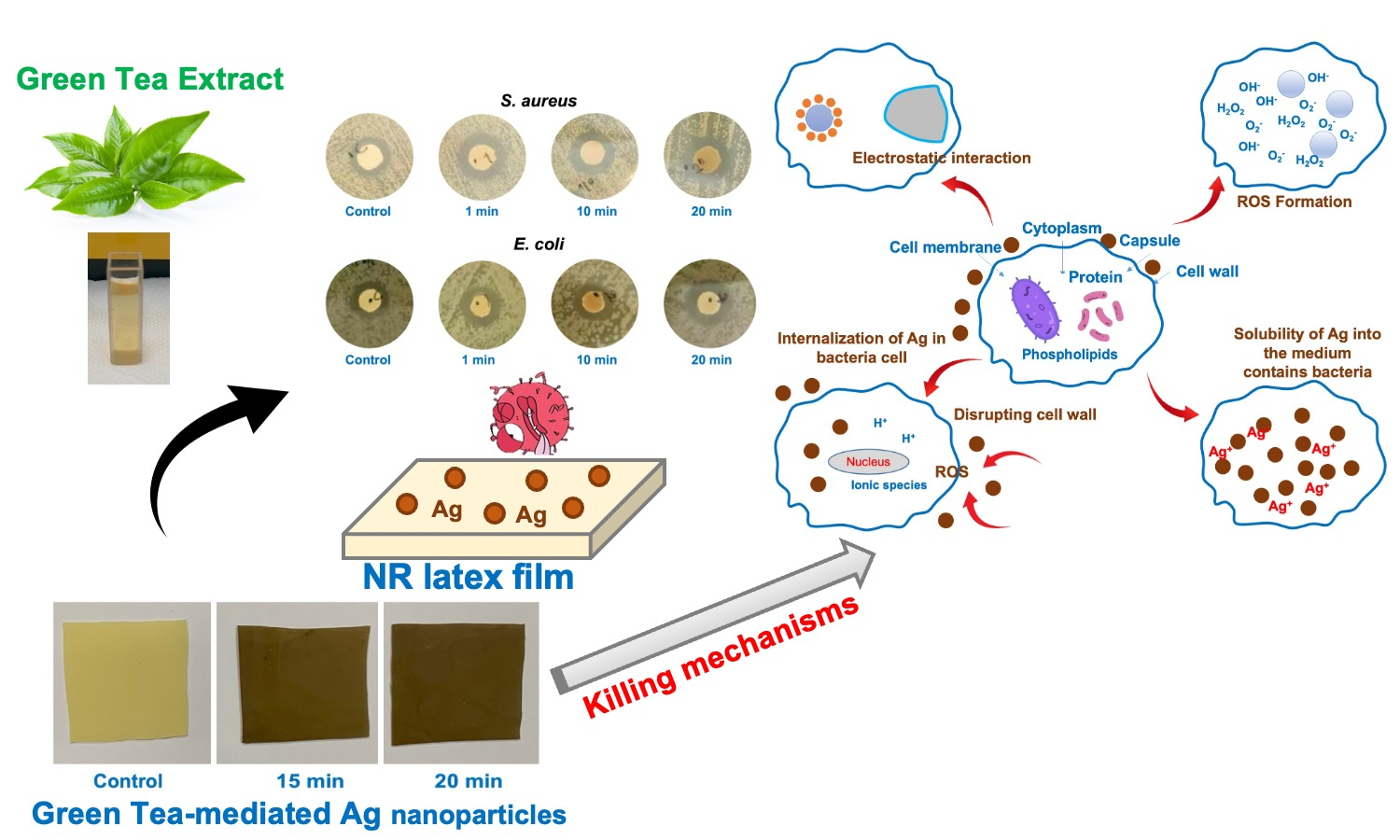
An antibacterial natural rubber (NR) latex film was successfully prepared in this study. This was done by coating silver (Ag) nanoparticles onto the surface of the NR latex film. The Ag nanoparticles were synthesized using green tea (GT) extract as a bio-reducing agent. The corresponding Ag nanoparticles were then deposited onto the NR latex film. Before synthesis, the phenolic compounds were identified using high-performance liquid chromatography (HPLC). The Ag nanoparticles were found to be smaller than 25 nm in size. Subsequently, an experimental evaluation was conducted to determine the influence of deposition time, namely 1 to 20 min, on the film’s overall performance. Scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (SEM-EDX) confirmed that the Ag content was higher over the deposition time. The surface roughness of the samples was also screened by atomic force microscopy (AFM), where the films became rougher over the deposition time, confirming that Ag nanoparticles dispersed over the surface. As for the antibacterial activities, both qualitative and quantitative tests showed significant outputs. The clear zones of S. aureus and E. coli increased over the deposition time, and a shorter contact time was used to kill the bacteria. This study offers a scientific foundation that supports the development of future rubber products utilizing these findings.
Jose James, George Vazhathara Thomas, Sisanth Krishnageham Sidharathan, Mohammad Arif Poothanari, Sabu Thomas
Vol. 19., No.7., Pages 697-705, 2025
DOI: 10.3144/expresspolymlett.2025.53
Vol. 19., No.7., Pages 697-705, 2025
DOI: 10.3144/expresspolymlett.2025.53
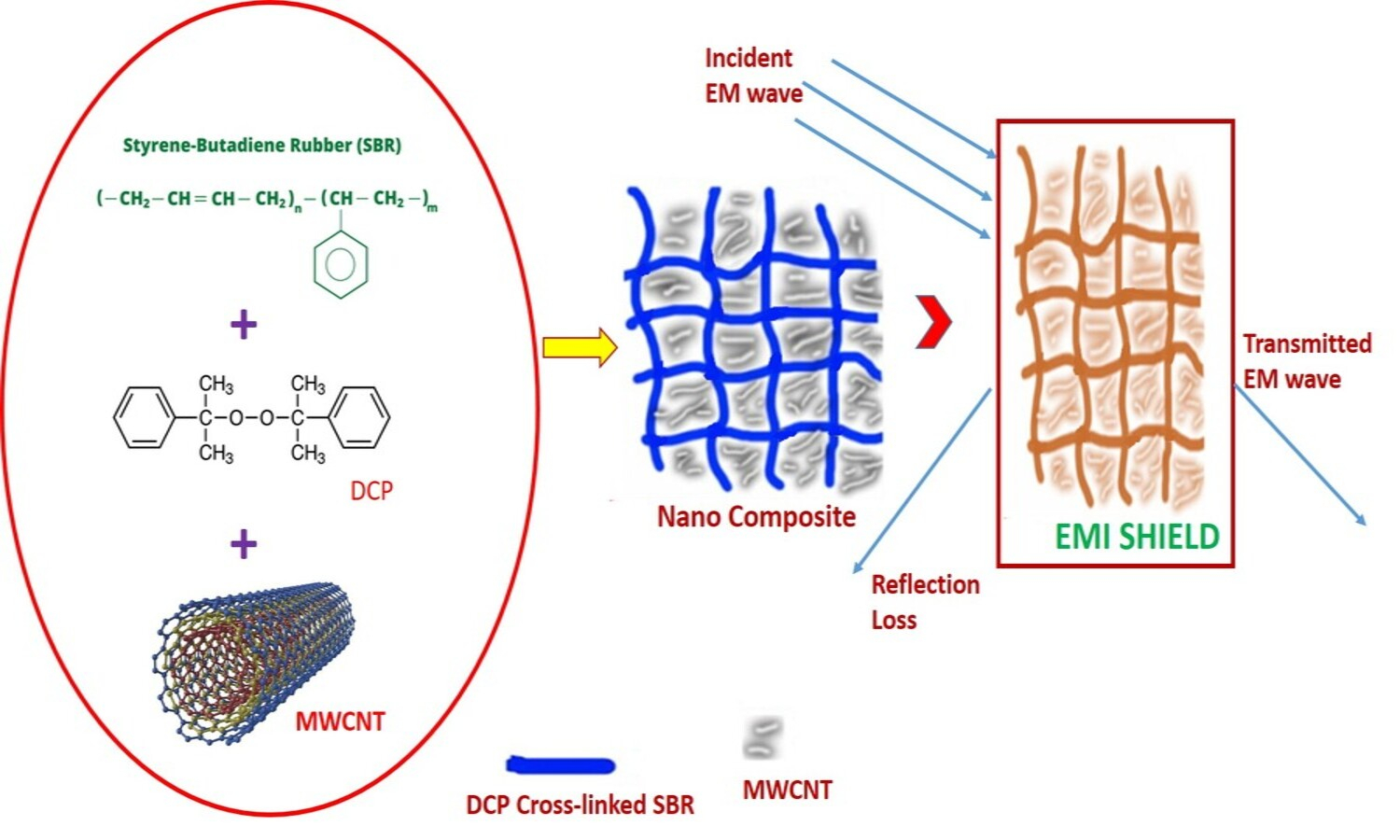
A nanocomposite of styrene butadiene rubber (SBR) and multi-walled carbon nanotubes (MWCNT) was fabricated using an internal melt mixer. Systematically investigated the role of MWCNT loading on the mechanical, dielectric, electrical and Electromagnetic interference (EMI) shielding characteristics of developed nanocomposites. The fine dispersion of MWCNTs in the SBR matrix was clearly observed from high-resolution transmission electron microscope images. The nanocomposites exhibited outstanding electrical, dielectric and EMI shielding behaviours (~45 dB at 20 phr of MWCNT). A high conductivity of 0.92 S/cm was attained in the nanocomposites and is attributable to the establishment of percolation networks of MWCNT in the SBR matrix. These composites displayed reasonably good mechanical properties because of the reinforcing effect of MWCNT. The economically viable and easy fabrication protocol of this nanocomposite can act as a platform for the synthesis of low-cost and highly effective composite for EMI shielding applications.
Wenxin Gan, Hanyu Xue, Hongyi Lin, Renjin Gao, Yuchi Zhang, Liwei Wang, Jiuping Rao
Vol. 19., No.3., Pages 311-325, 2025
DOI: 10.3144/expresspolymlett.2025.22
Vol. 19., No.3., Pages 311-325, 2025
DOI: 10.3144/expresspolymlett.2025.22
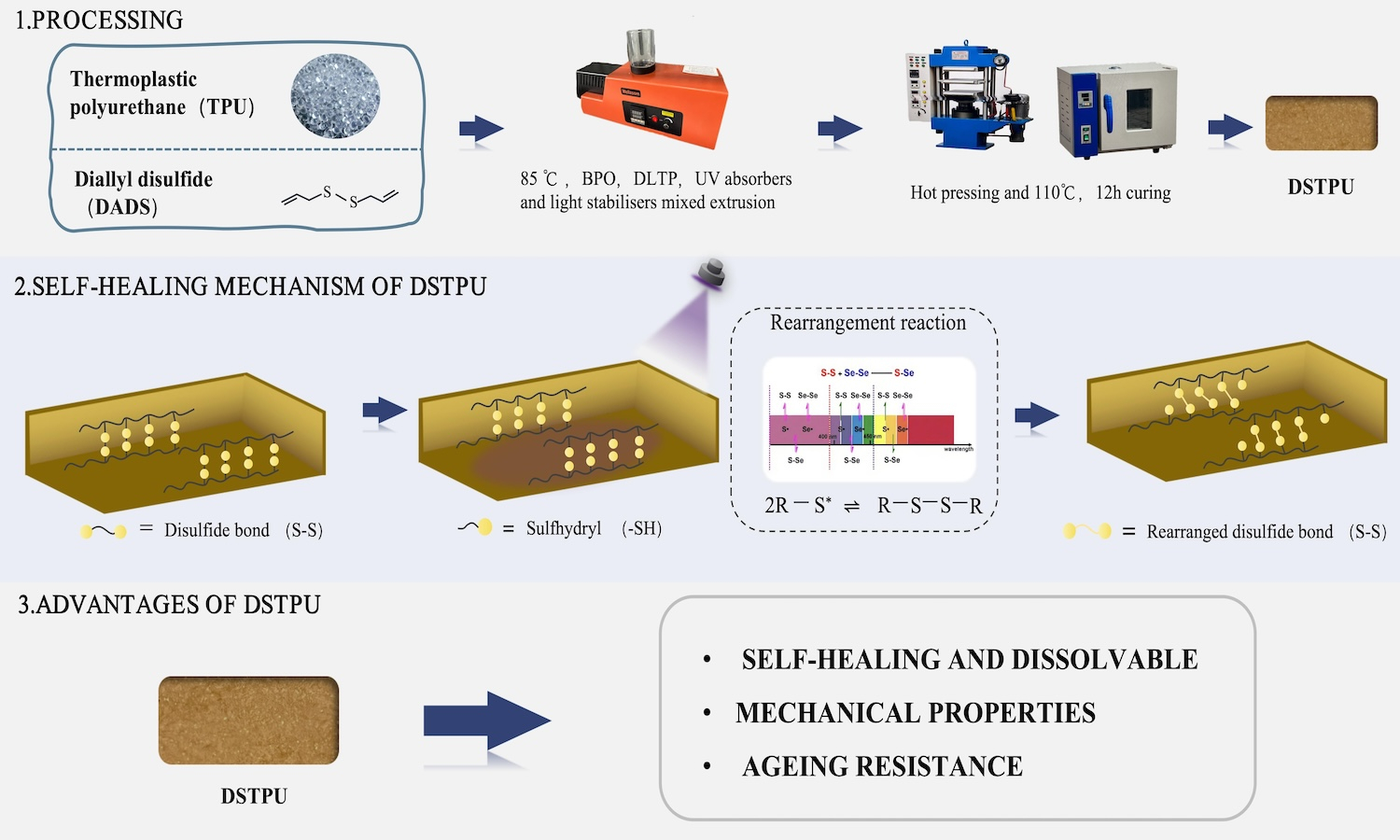
Cross-linking frequently enhanced the mechanical properties of linear polymeric materials; however, it also resulted in the transition from thermoplastic to thermosetting materials, which posed issues from an environmental perspective. Thermoplastic polyurethane (TPU) elastomers were extensively applied across various industries. To improve the mechanical properties of TPU while preserving its environmental benefits, this study integrated radical copolymerization technology to develop a reversible crosslinked TPU. Specifically, the linear polyurethane molecular chains were crosslinked using diallyl disulfide (DADS) as a functional cross-linking monomer. Through radical copolymerization reactions, reversible crosslinks formed from disulfide bonds were created between the linear polyurethane molecular chains, yielding a self-healing reversible crosslinked thermoplastic polyurethane (DSTPU). The study showed that DSTPU could self-heal and dissolve under UV light and alkaline N,N-dimethylformamide (DMF) conditions, achieving 82.2% self-healing efficiency at 3 phr DADS. It dissolved into fine particles in alkaline DMF. Disulfide bonds in DSTPU enhanced cross-linking, boosting 19% oxygen permeability, thermal conductivity (0.218 W/(m·K)), and mechanical properties like tensile stress (11.18 MPa), force (134.13 N), and elongation (548%). These bonds also enhanced aging resistance, cutting ΔYI to 6.0%.




