Curing, rheological, mechanical, and flame retardant properties of high thermal-resistant dibutyl phosphate-bound natural rubber
Vol. 18., No.6., Pages 623-637, 2024
DOI: 10.3144/expresspolymlett.2024.46
DOI: 10.3144/expresspolymlett.2024.46
GRAPHICAL ABSTRACT

ABSTRACT
Dibutyl phosphate-bound natural rubber (DBNR) was prepared by reacting epoxidized natural rubber with 20 mol% (ENR-20) with dibutyl phosphate in a latex medium. Fourier transform infrared spectroscopy (FTIR) was used to confirm the molecular structures of ENR-20 and DBNR and to quantify the epoxide contents. The shear flows and thermal properties of DBNR were then characterized and compared with ENR-20 and natural rubber (NR). The DBNR exhibited the lowest viscosity curves, but it depicted the highest glass transition temperature (Tg) and residue from thermogravimetric evaluation, indicating higher thermal resistance. Subsequently, different NR/DBNR blend ratios were compounded, with and without flame resistance additives, compared with unmodified NR and chloroprene rubber (CR) compounds. We found that all natural rubber compounds exhibited reversion behavior due to the breakage of newly formed sulfidic bonds. However, chloroprene rubber showed marching cured curves, as evidenced by the increasing torque with prolonged testing time. Additionally, antimony trioxide retarded the curing reaction of NR, while tris(2-ethylhexyl) phosphate accelerated it. Therefore, the combination of these additives synergists with the intrinsic flame retardant properties of DBNR. The study revealed that the burning rate of NR/DBNR blends, exhibited very high flame resistance capability compared to gum NR and NR compounded with flame resistance additives.
RELATED ARTICLES
Reinforcing effect of thermo-oxidative reclaimed rubber on NR/SBR blends for tire tread applications
Yunhui Xu, Zaheer ul Haq, Junrong Li, Hui Tu, Zaixue Wang, Houluo Cong
Vol. 20., No.2., Pages 142-153, 2026
DOI: 10.3144/expresspolymlett.2026.12
Vol. 20., No.2., Pages 142-153, 2026
DOI: 10.3144/expresspolymlett.2026.12
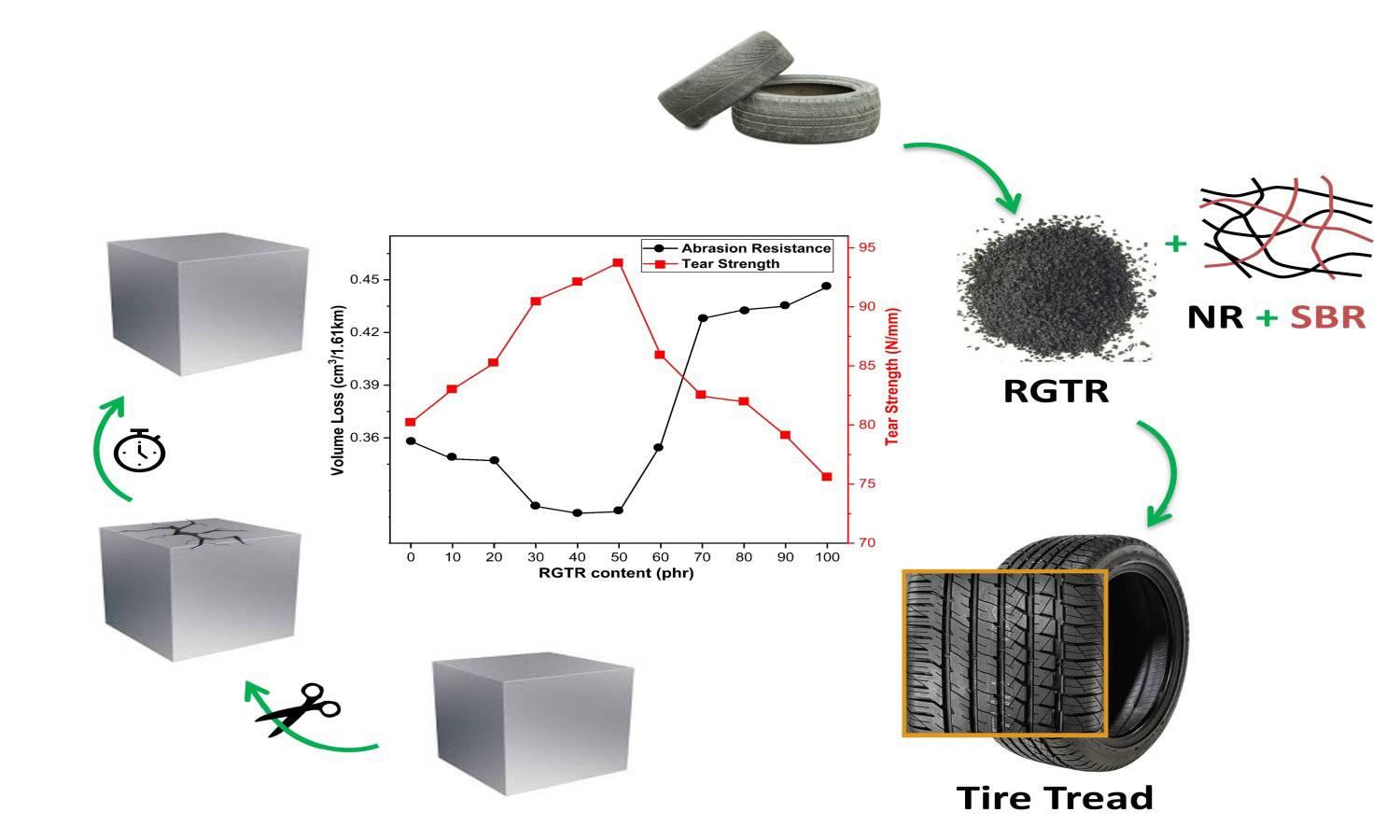
This study explores the application of thermo-oxidative reclaimed ground tire rubber (RGTR) in natural rubber (NR)/styrene butadiene rubber (SBR) composite, focusing on its impact on morphology, mechanical properties, rheological behavior, vulcanization characteristics, aging resistance, tear strength and abrasion resistance. The findings revealed that RGTR enhances the tear strength and abrasion resistance of NR/SBR composites while maintaining comparable tensile strength, elongation at break, and modulus. The incorporation of RGTR reduced Mooney viscosity of the NR/SBR composites and improved flowability. It also shortened the vulcanization time and enhanced vulcanization efficiency. The NR/SBR composites with RGTR loadings below 60 phr exhibited optimal performance, achieved a maximum tear strength of 93.77 N/mm and improved abrasion resistance. However, higher RGTR content led to increased agglomeration, as evidenced by scanning electron microscopy (SEM), which showed finer dispersion at lower RGTR contents and larger aggregates at higher loadings. These findings demonstrate the potential of RGTR as a sustainable additive for enhancing specific properties in NR/SBR composites, contributing to both performance optimization and waste tire management.
Rattanawadee Ninjan, Bencha Thongnuanchan, Phakawat Tongnuanchan, Subhan Salaeh, Jutharat Intapun, Abdulhakim Masa, Natinee Lopattananon
Vol. 20., No.1., Pages 18-35, 2026
DOI: 10.3144/expresspolymlett.2026.3
Vol. 20., No.1., Pages 18-35, 2026
DOI: 10.3144/expresspolymlett.2026.3
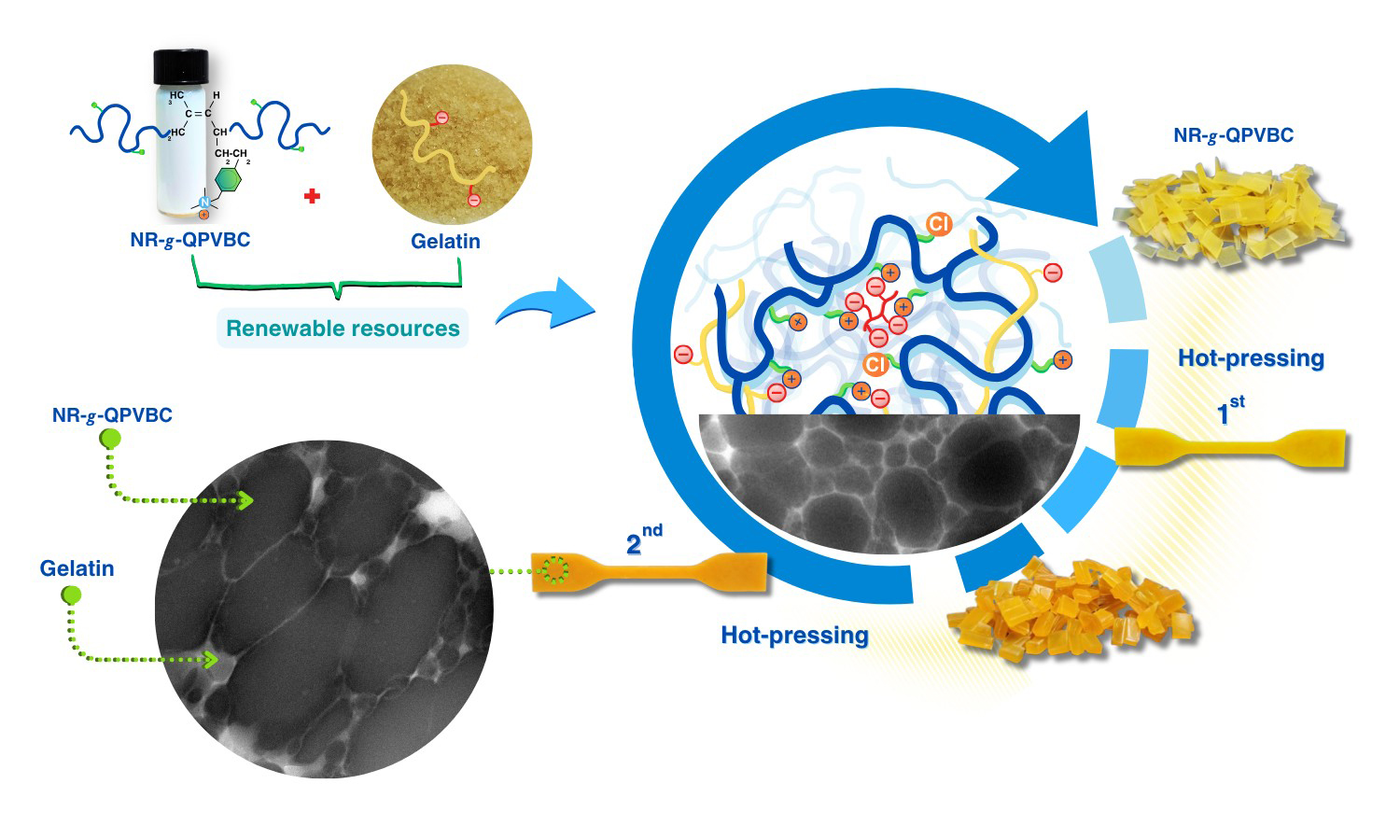
The present study has proposed a straightforward method to improve the reprocessability of modified natural rubber (NR) by blending it with gelatin (GT). The reprocessable characteristics of these blends were evaluated based on their remolding capabilities and mechanical recovery performance. In this method, poly(vinylbenzyl chloride) (PVBC) was first grafted onto NR chains to create graft copolymers known as NR-g-PVBC. The benzyl chloride groups in the graft copolymers were subsequently converted into quaternary ammonium groups, referred to as NR-g-QPVBC. This modification enabled ionic crosslinking when NR-g-QPVBC reacted with ethylenediamine tetraacetic acid. Blends were created by incorporating GT powder into the NR-g-QPVBC latex. The optimal loading level of GT was determined to be 30 wt%, as the resulting film exhibited the highest recovery of tensile properties. Initially, the film's tensile strength was measured at 15 MPa. After being remolded at 160 °C, the tensile strength decreased to 9.3 MPa, resulting in a recovery rate of 60.7% and withstanding a tensile strain of 144%. Although the NR-g-QPVBC/GT films could be remolded, their tensile properties declined with increasing remolding cycles. Therefore, this work demonstrated a practical method for producing NR-based films that could be reshaped through hot-pressing after being formed into products, increasing their reusability.
Azizon Kaesaman, Tassaneeya Khunrang, Charoen Nakason
Vol. 19., No.8., Pages 753-772, 2025
DOI: 10.3144/expresspolymlett.2025.58
Vol. 19., No.8., Pages 753-772, 2025
DOI: 10.3144/expresspolymlett.2025.58
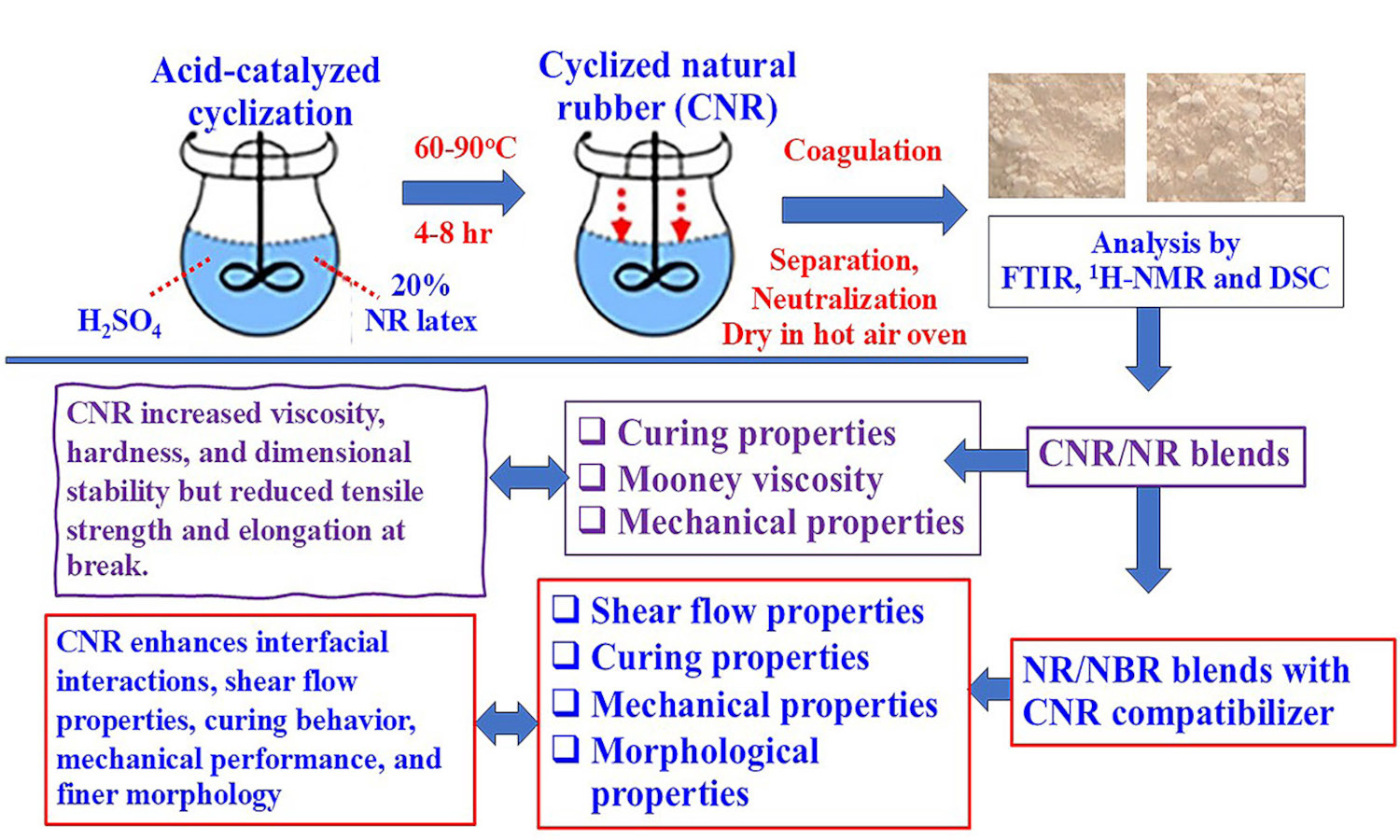
Cyclized natural rubber (CNR) was synthesized through the acid-catalyzed reaction of natural rubber (NR) latex using sulfuric acid as a catalyst and stabilized with a non-ionic surfactant. Cyclization was evaluated by iodine numbers under varying reaction times, temperatures, and NR-to-acid ratios. Fourier transform infrared spectroscopy (FTIR) and proton nuclear magnetic resonance spectroscopy (1H-NMR) confirmed the formation of cyclic structures in CNR molecules. Differential scanning calorimetry (DSC) showed that the glass transition temperature (Tg) of CNR increased with cyclization, indicating greater rigidity and less chain flexibility. CNR was then blended with NR and used as a compatibilizer in NR/acrylonitrile butadiene rubber (NBR)blends. It increased blend viscosity, hardness, and dimensional stability but reduced tensile strength and elongation due to its rigid cyclic domains. In NR/NBR blends, CNR outperformed a commercial homogenizer in enhancing interfacial interactions, leading to superior shear flow properties, curing behavior, and mechanical performance. This is attributed to the polar groups in CNR, which enhance intermolecular interactions and phase compatibility, resulting in finer phase morphology. This study highlights the potential of CNR as a versatile material for enhancing the performance of rubber compounds, with promising applications in advanced industrial formulations.
Abdulhakim Masa, Nureeyah Jehsoh, Sawitree Dueramae, Nabil Hayeemasae
Vol. 19., No.7., Pages 653-669, 2025
DOI: 10.3144/expresspolymlett.2025.50
Vol. 19., No.7., Pages 653-669, 2025
DOI: 10.3144/expresspolymlett.2025.50
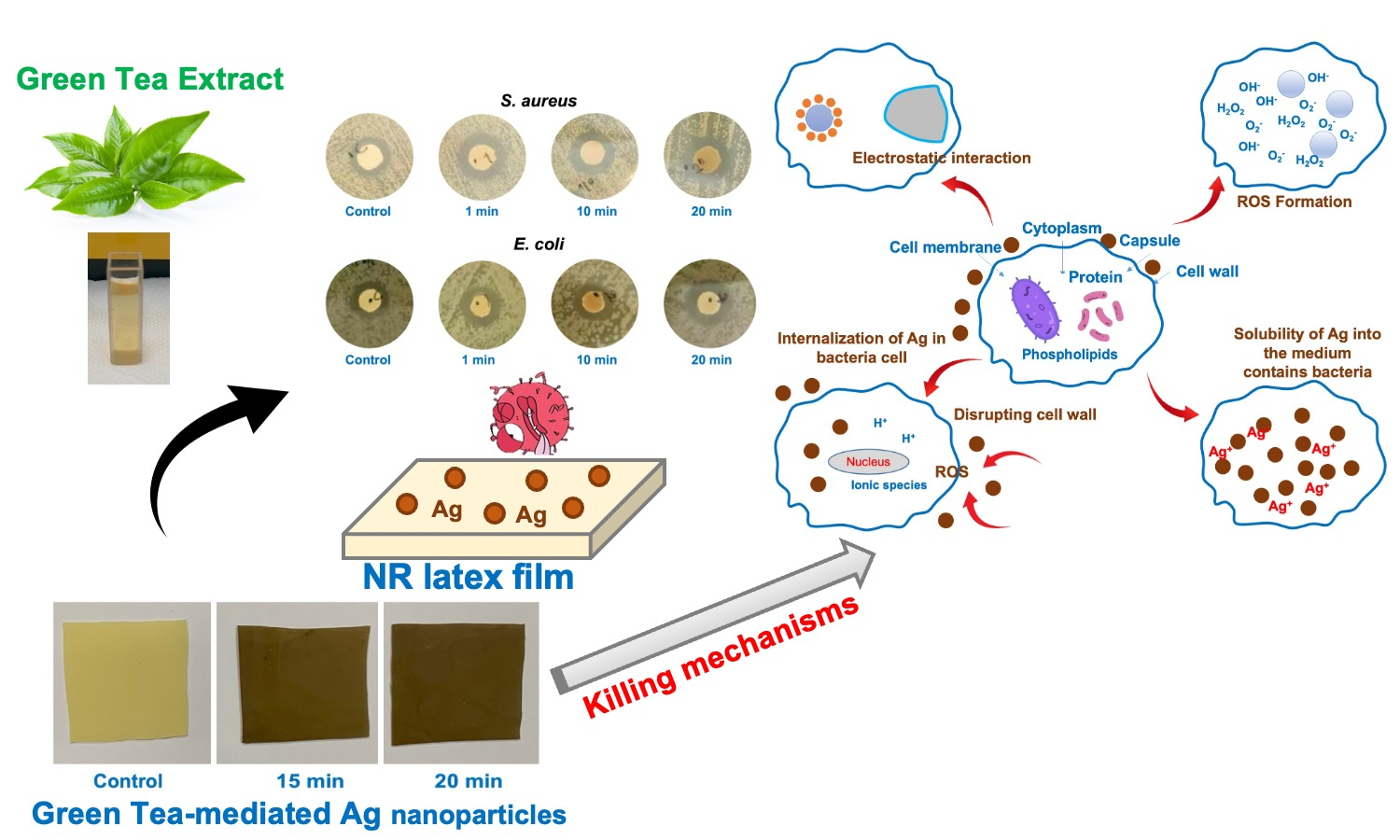
An antibacterial natural rubber (NR) latex film was successfully prepared in this study. This was done by coating silver (Ag) nanoparticles onto the surface of the NR latex film. The Ag nanoparticles were synthesized using green tea (GT) extract as a bio-reducing agent. The corresponding Ag nanoparticles were then deposited onto the NR latex film. Before synthesis, the phenolic compounds were identified using high-performance liquid chromatography (HPLC). The Ag nanoparticles were found to be smaller than 25 nm in size. Subsequently, an experimental evaluation was conducted to determine the influence of deposition time, namely 1 to 20 min, on the film’s overall performance. Scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (SEM-EDX) confirmed that the Ag content was higher over the deposition time. The surface roughness of the samples was also screened by atomic force microscopy (AFM), where the films became rougher over the deposition time, confirming that Ag nanoparticles dispersed over the surface. As for the antibacterial activities, both qualitative and quantitative tests showed significant outputs. The clear zones of S. aureus and E. coli increased over the deposition time, and a shorter contact time was used to kill the bacteria. This study offers a scientific foundation that supports the development of future rubber products utilizing these findings.
Marek Pöschl, Radek Stoček, Petr Zádrapa, Martin Stěnička, Gert Heinrich
Vol. 18., No.12., Pages 1178-1190, 2024
DOI: 10.3144/expresspolymlett.2024.90
Vol. 18., No.12., Pages 1178-1190, 2024
DOI: 10.3144/expresspolymlett.2024.90
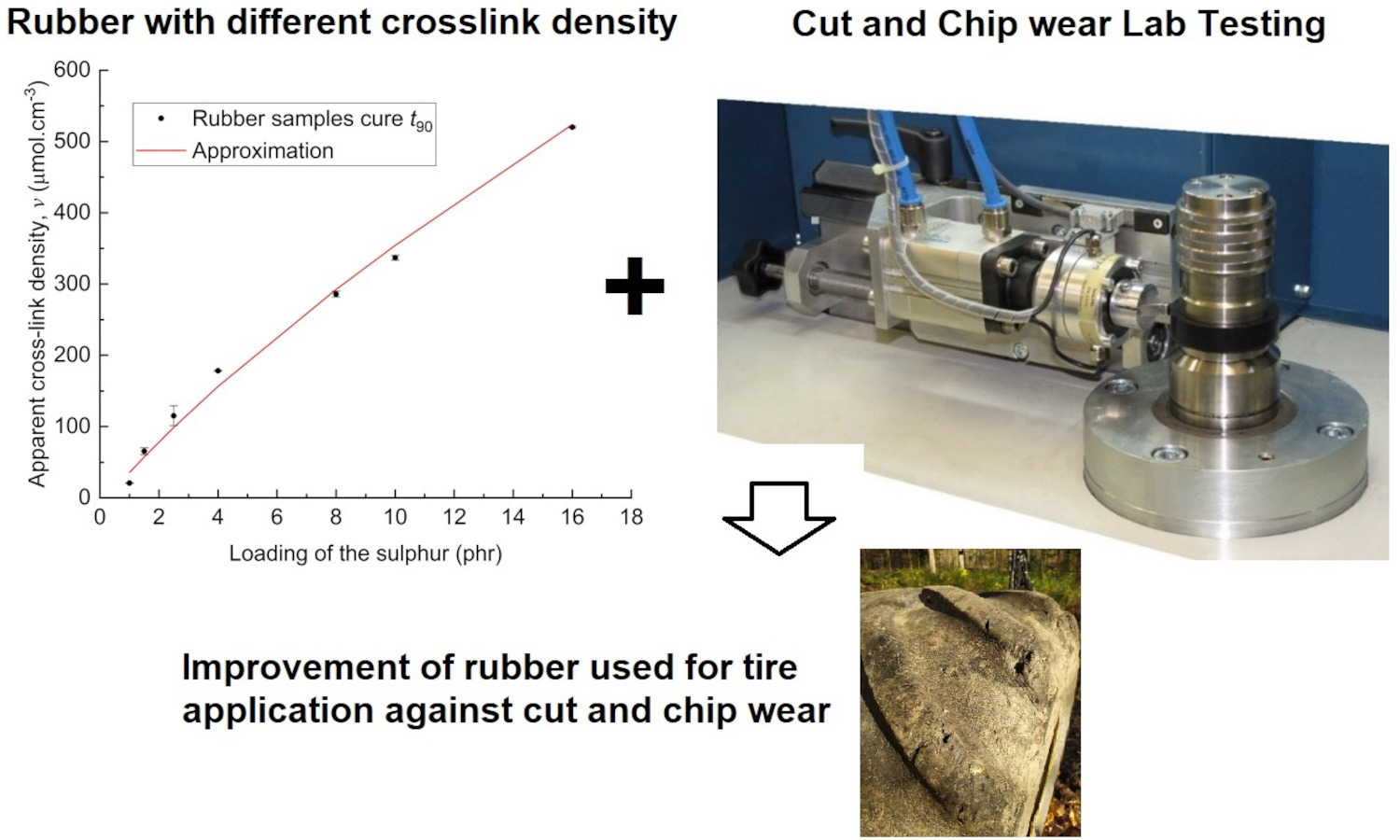
This paper extends previous studies by the authors that aimed to describe the effect of apparent cross-link density (CLD) of the rubber polymer networks on the fracture mechanism caused by cut and chip (CC) wear of natural rubber (NR), demonstrating the positive effect of conventional vulcanization (CV). This work is focused on the determination of the effect of CLD while keeping constant the accelerator-to-sulfur ratio A/S = 0.2, typical for CV systems. For this ratio, different sulfur quantities were chosen, and the concentration of the accelerator N-tert-butyl-benzothiazole sulphonamide (TBBS) was calculated to achieve CLDs in a range from 35 to 524 μmol・cm–3. Standard analyses such as tensile tests, hardness, rebound resilience and DIN abrasion were performed. From these analyses, the optimum physical properties of the NR-based rubber were estimated to be in the CLD range of approximately 60 to 160 μmol・cm–3. A CC wear analysis was performed with an Instrumented cut and chip analyzer (ICCA) and it was found that the highest CC wear resistance of the NR is in the CLD range of 35 to 100 μmol・cm–3. Furthermore, the effect of straininduced crystallization (SIC) of NR on CC wear and its dependence on the CLD region was discussed. For the first time, we determine a CLD range for a CV system in which the material achieves both optimal mechanical properties and CC wear resistance.




