Potential for using sepiolite as dispersing agent in phenolic resin crosslinked natural rubber/silica composites
Nabil Hayeemasae , Sitisaiyidah Saiwari
, Sitisaiyidah Saiwari , Siriwat Soontaranon
, Siriwat Soontaranon , Mohamad Irfan Fathurrohman
, Mohamad Irfan Fathurrohman , Abdulhakim Masa
, Abdulhakim Masa
 , Sitisaiyidah Saiwari
, Sitisaiyidah Saiwari , Siriwat Soontaranon
, Siriwat Soontaranon , Mohamad Irfan Fathurrohman
, Mohamad Irfan Fathurrohman , Abdulhakim Masa
, Abdulhakim Masa
Vol. 19., No.3., Pages 339-349, 2025
DOI: 10.3144/expresspolymlett.2025.24
DOI: 10.3144/expresspolymlett.2025.24
GRAPHICAL ABSTRACT
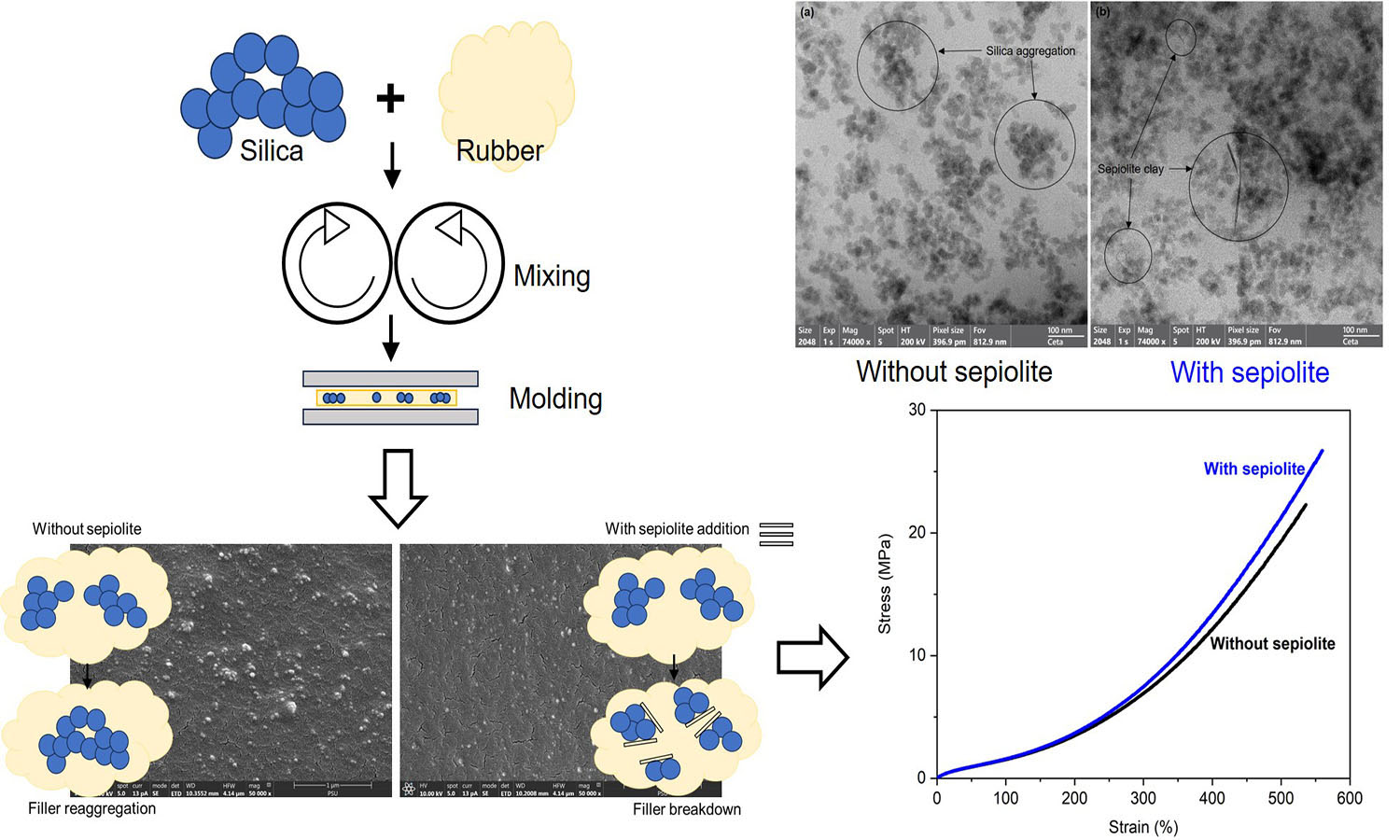
ABSTRACT
Natural rubber (NR) composites filled with silica and crosslinked with phenolic resin were prepared in this study. The influence of a small sepiolite addition (1–5 part(s) per hundred parts of rubber, phr) on the properties of NR composites was studied. It was found that sepiolite reduced silica aggregate size, allowing improved dispersion in the NR matrix. Sepiolite facilitates silica dispersion by locating at the silica surfaces and acting as a barrier that prevents agglomeration of silica filler. The swelling resistance, crosslink density, tensile strength, and strain-induced crystallization were all strengthened by incorporating sepiolite because of the improved silica dispersion. The greatest tensile strength was achieved at a 2 phr sepiolite addition level. The improvement was about 18% over the reference composite due to the greatest filler-rubber interactions and the finest filler dispersion. The results clearly indicate that sepiolite clay can be applied as a dispersing agent in silica-containing rubber composites.
RELATED ARTICLES
Wenhui Han, Yaqi Ge, Peng Wang, Haojun Zang, Shengqiang Xu, Huiguang Bian, Chuansheng Wang
Vol. 19., No.12., Pages 1274-1285, 2025
DOI: 10.3144/expresspolymlett.2025.94
Vol. 19., No.12., Pages 1274-1285, 2025
DOI: 10.3144/expresspolymlett.2025.94

This study investigates eugenol as an alternative to mitigate environmental pollution and worker hazards associated with antioxidant N-(1,3-dimethyl)butyl-N′-phenyl-p-phenylenediamine (4020) while aligning with trends toward sustainable additives. Silica/natural rubber (NR) composites with varying ratios of eugenol and 4020 were prepared to assess their aging resistance, mechanical properties, and the synergistic antioxidant effects. Thermogravimetric analysis, cross-linking density experiments, thermo-oxidative aging tests, and oxidation induction tests revealed the highest thermo-oxidative aging resistance when 0.5 phr of 4020 was substituted with eugenol. When 1.0 phr of 4020 was replaced by eugenol, the antioxidant properties of the composites matched those containing 2.0 phr of 4020. However, when eugenol exceeded 1 phr, the antioxidant properties gradually decreased. DIN wear tests showed optimal wear resistance when 1 phr of 4020 was replaced with eugenol. These findings suggest that 50% of conventional antioxidants can be substituted with eugenol without compromising material properties. The partial substitution of eugenol in silica/NR composites proves eugenol can act as a sustainable alternative, providing comparable antioxidant capacity while reducing environmental impact.
Jutatip Makmanee Treitler, Diew Saijun, Kritsada Phatcharasit, Suwat Rattanapan
Vol. 19., No.12., Pages 1310-1319, 2025
DOI: 10.3144/expresspolymlett.2025.96
Vol. 19., No.12., Pages 1310-1319, 2025
DOI: 10.3144/expresspolymlett.2025.96
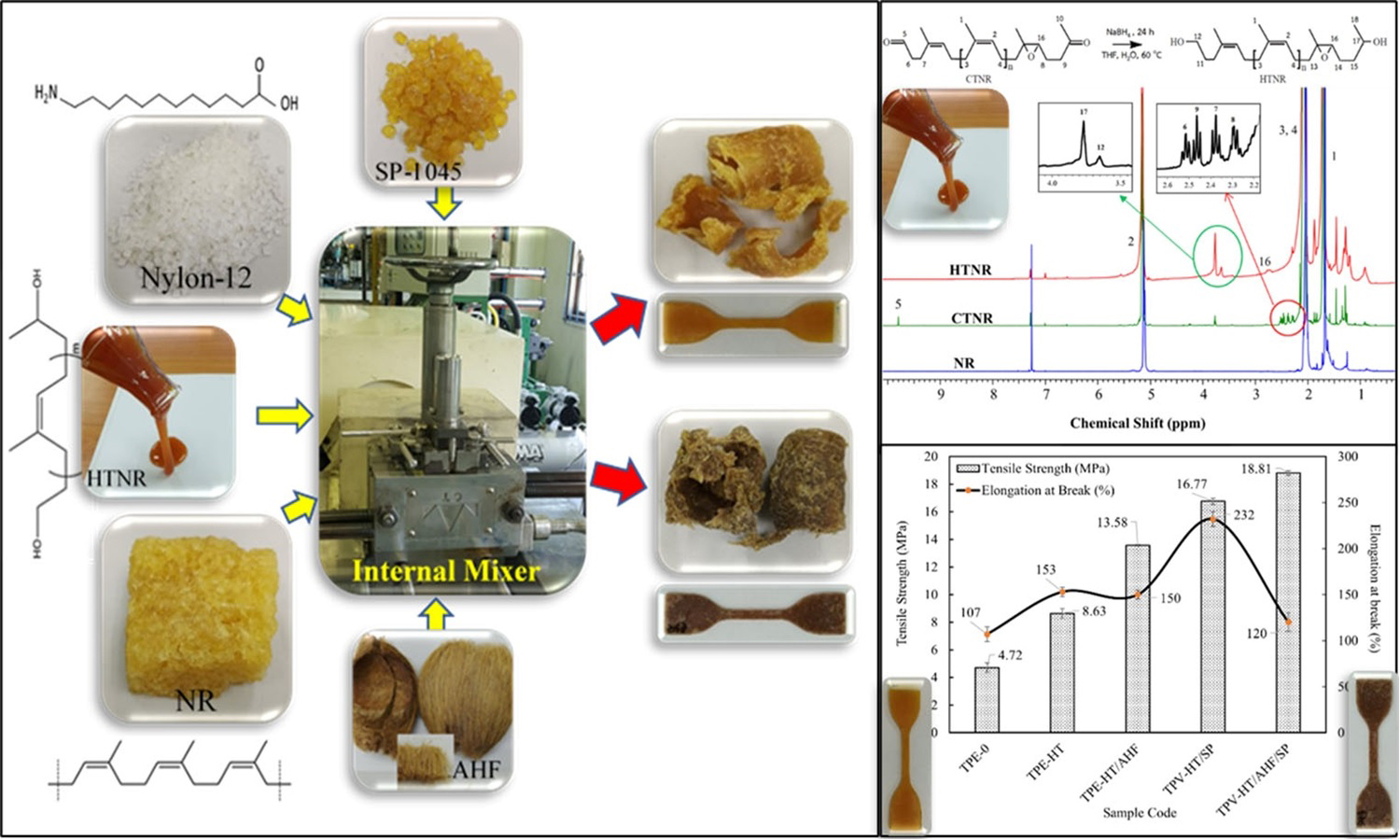
This work introduces an innovative method to enhance the compatibility of nylon-12/natural rubber thermoplastic elastomers by utilizing hydroxyl telechelic natural rubber as a reactive compatibilizer and natural fibers as reinforcement. Hydroxyl telechelic natural rubber was synthesized from natural rubber via oxidative cleavage to carbonyl telechelic natural rubber, followed by reduction with sodium borohydride. Proton nuclear magnetic resonance (1H-NMR) and Fourier transform infrared spectroscopy (FTIR) verified the structure. Incorporating hydroxyl telechelic natural rubber into nylon-12/natural rubber (40/60 wt%) blends significantly enhanced interfacial adhesion, improving tensile strength and elongation at break compared to the uncompatibilized mix. Dynamic vulcanization using phenolic resin achieved an optimal balance of strength and ductility. The incorporation of areca husk fiber enhanced tensile strength, hardness, and solvent resistance, with a slight decrease in ductility and tear strength. Rheological analysis indicated that hydroxyl telechelic natural rubber increased melt viscosity due to improved phase interactions, while dynamic vulcanization reduced the melt flow index through network formation. Solvent uptake experiments confirmed that hydroxyl telechelic natural rubber, areca husk fiber, and SP-1045 vulcanizing agent minimized swelling in isooctane, toluene, and diesel oil.
Kinsuk Naskar
Vol. 19., No.10., Pages 977-978, 2025
DOI: 10.3144/expresspolymlett.2025.73
Vol. 19., No.10., Pages 977-978, 2025
DOI: 10.3144/expresspolymlett.2025.73

This is an editorial article. It has no abstract.
Sirithorn Kaewklum, Parisa Faibunchan, Apinya Krainoi, Banyat Cherdchim, Jutharat Intapun
Vol. 19., No.9., Pages 929-945, 2025
DOI: 10.3144/expresspolymlett.2025.70
Vol. 19., No.9., Pages 929-945, 2025
DOI: 10.3144/expresspolymlett.2025.70
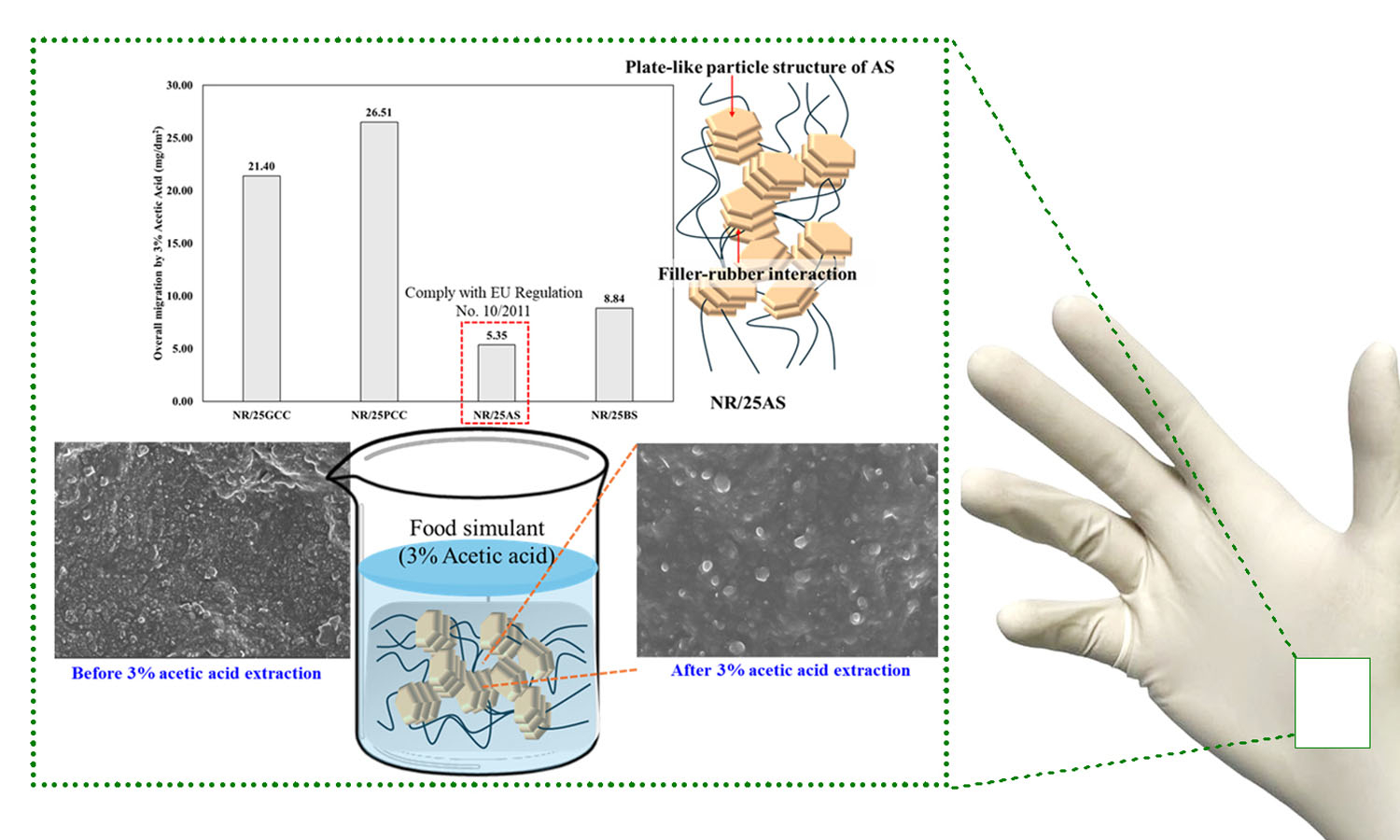
Powder-free natural rubber gloves for chemical migration resistance of food-contact grade are prepared using a variety of fillers, including ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), aluminum silicate (AS), and barium sulfate (BS)-filled natural rubber (NR), respectively. The properties of NR gloves, including mechanical, dynamic mechanical, and thermal properties, were investigated. Furthermore, the overall migration test of NR gloves was conducted according to the regulations for food contact gloves (EU Regulation No. 10/2011), using 3% acetic acid as the simulant. Among the fillers studied, the plate-like particles of AS facilitated the most effective filler-rubber interactions and reinforcement in AS-filled natural rubber (NR/AS). Consequently, the highest crosslink density, force at break, and damping properties of NR gloves were achieved by applying AS in the NR matrix. Moreover, the lowest overall migration level was observed for NR/AS with a value of 5.35 mg/dm2, which complies with EU Regulation (overall migration of food simulants shall not exceed 10 mg/dm2). Therefore, NR gloves filled with AS are suitable for food-contacting NR gloves.
Azizon Kaesaman, Tassaneeya Khunrang, Charoen Nakason
Vol. 19., No.8., Pages 753-772, 2025
DOI: 10.3144/expresspolymlett.2025.58
Vol. 19., No.8., Pages 753-772, 2025
DOI: 10.3144/expresspolymlett.2025.58
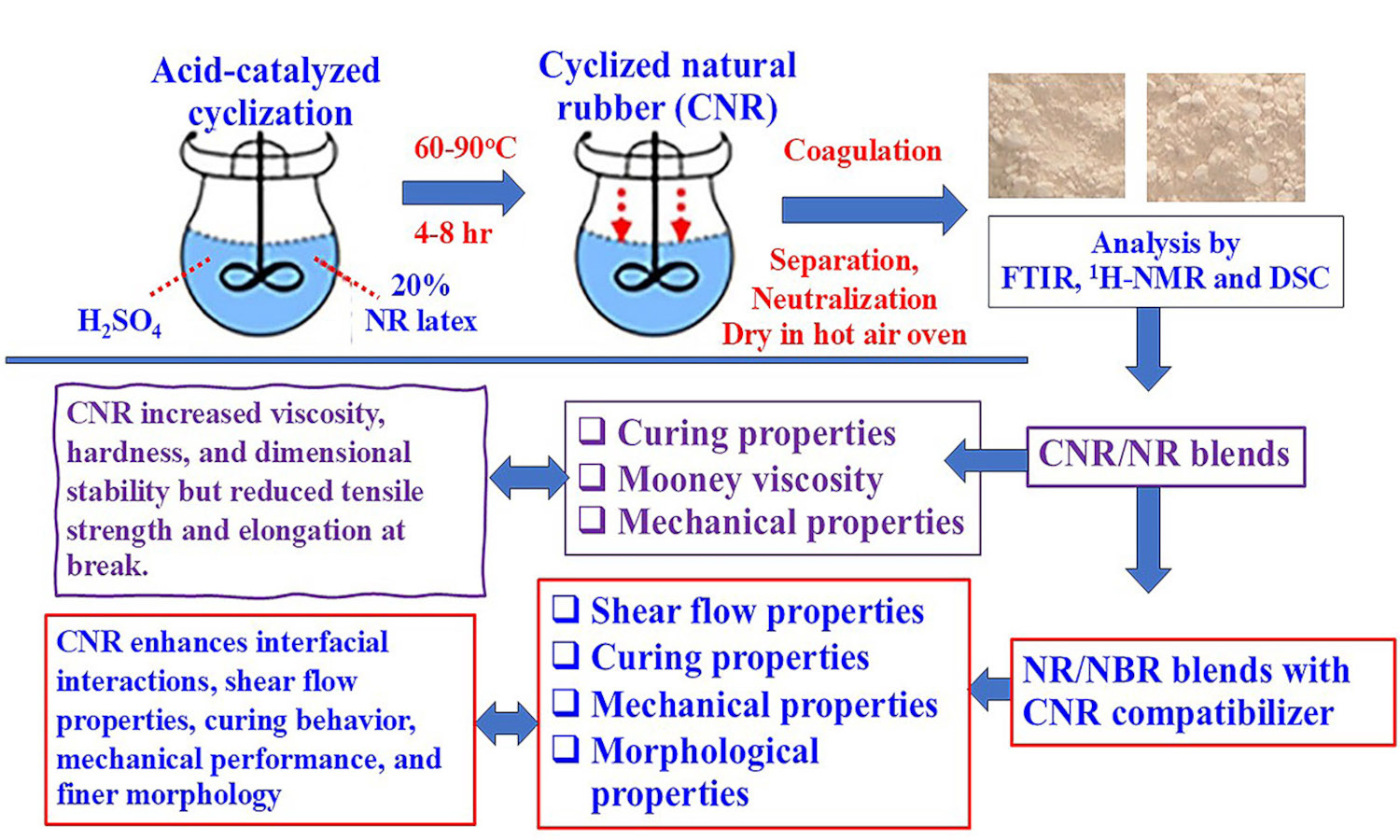
Cyclized natural rubber (CNR) was synthesized through the acid-catalyzed reaction of natural rubber (NR) latex using sulfuric acid as a catalyst and stabilized with a non-ionic surfactant. Cyclization was evaluated by iodine numbers under varying reaction times, temperatures, and NR-to-acid ratios. Fourier transform infrared spectroscopy (FTIR) and proton nuclear magnetic resonance spectroscopy (1H-NMR) confirmed the formation of cyclic structures in CNR molecules. Differential scanning calorimetry (DSC) showed that the glass transition temperature (Tg) of CNR increased with cyclization, indicating greater rigidity and less chain flexibility. CNR was then blended with NR and used as a compatibilizer in NR/acrylonitrile butadiene rubber (NBR)blends. It increased blend viscosity, hardness, and dimensional stability but reduced tensile strength and elongation due to its rigid cyclic domains. In NR/NBR blends, CNR outperformed a commercial homogenizer in enhancing interfacial interactions, leading to superior shear flow properties, curing behavior, and mechanical performance. This is attributed to the polar groups in CNR, which enhance intermolecular interactions and phase compatibility, resulting in finer phase morphology. This study highlights the potential of CNR as a versatile material for enhancing the performance of rubber compounds, with promising applications in advanced industrial formulations.



